Imaging brain waste clearance in neurodegenerative diseases
Abstract
Cerebral waste clearance has emerged as a pivotal research focus in global neuroscience, particularly involving neurodegenerative diseases (NDs). Extensive preclinical studies suggest that impaired waste clearance mechanisms may significantly contribute to the pathogenesis of these diseases. With brain aging, multiple interacting factors, such as vascular dysfunction, sleep disturbances, and neuroinflammation, may collectively compromise waste clearance efficiency. This dysfunction leads to pathological protein accumulation, oligomerization, and subsequent deposition. These insights unveil exciting opportunities for redefining the mechanisms underlying NDs and for developing novel therapeutic strategies. However, critical knowledge gaps persist: The cerebral clearance system comprises multiple complementary pathways with intricate sub-processes, yet the precise processes impaired in human neurodegenerative diseases and their relative contributions to disease progression remain unclear. In vivo imaging methods are essential tools for investigating these fundamental questions in human subjects. This commentary provides an updated overview of emerging in vivo imaging methodologies for assessing cerebral waste clearance dynamics, offering technical guidance to advance mechanistic studies and therapeutic development.
Keywords
INTRODUCTION TO BRAIN WASTE CLEARANCE
Like other human organs, cerebral cells generate diverse metabolic waste products during normal physiological processes, including carbon dioxide, lactate, proteinaceous debris, and cellular fragments. The timely clearance of these waste materials is essential, as their accumulation disrupts homeostasis, potentiates oxidative stress, promotes protein oligomerization and deposition, and ultimately drives neurodegeneration. Mounting evidence suggests a close association between impaired waste clearance and neurodegenerative diseases (NDs), such as Alzheimer’s disease (AD)[1] and Parkinson’s disease (PD)[2].
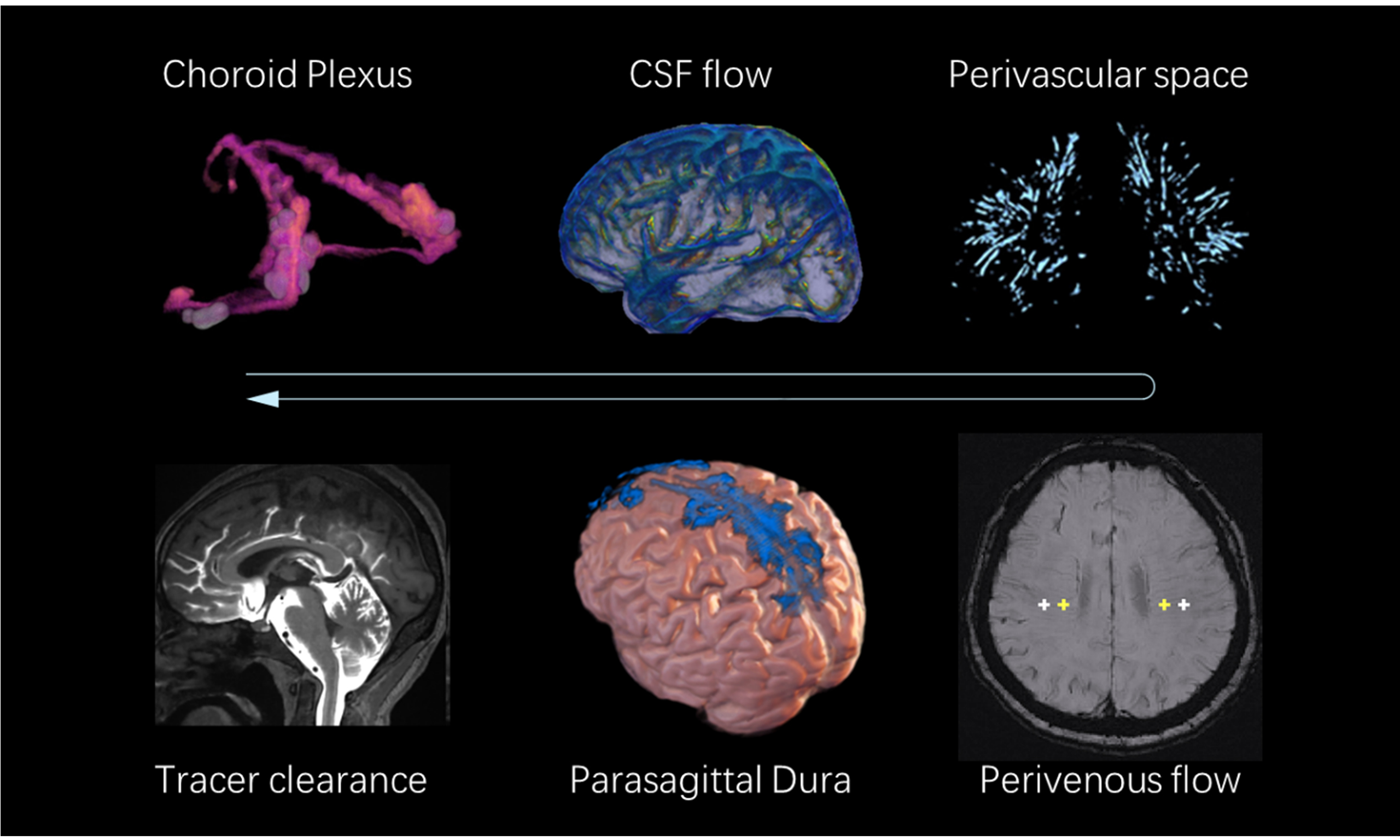
Human cerebral waste clearance is orchestrated through two interdependent systems: the vascular system and the cerebrospinal fluid (CSF) system[3]. In the vascular compartment, waste elimination primarily occurs through the blood-brain barrier (BBB)[4], which clears small molecules via both passive and active transport. In addition, the intramural periarterial drainage (IPAD) pathway facilitates clearance by retrograde transport within the basement membrane of cerebral arteries[5].
The CSF-mediated clearance system initiates with CSF production in the choroid plexus (ChP)[6]. After circulating through the ventricles, CSF traverses the subarachnoid space and enters periarterial spaces. Through aquaporin-4 (AQP4) water channels densely expressed on astrocytic endfeet, CSF infiltrates the brain parenchyma, mixes with interstitial fluid, and subsequently exits via AQP4-dependent efflux into perivenous spaces. Final drainage occurs either by absorption into the venous system via arachnoid granulations or by perineural pathways that connect to cervical lymph nodes. Notably, the blood-CSF barrier at the ChP epithelium serves as a key site for active solute exchange between these two systems[7].
Given the extensive existing literature detailing cerebral waste clearance pathways[8,9], we will not dive into the details in this review. Even from this brief overview, it becomes evident that cerebral waste clearance represents a highly complex biological process. While experimental models allow researchers to isolate variables for mechanistic investigation, human disease processes inherently involve multifaceted interactions across hierarchical levels. For instance, vascular degeneration may alter arterial pulsatility patterns, thereby compromising the driving force for CSF flow[10], whereas age-related sleep disturbances can impair interstitial fluid exchange by disrupting glymphatic flux[11]. Consequently, global glymphatic dysfunction may stem from diverse pathological mechanisms.
Significant knowledge gaps persist regarding how impairments in specific clearance routes and subprocesses combine to determine global waste clearance efficacy. More fundamentally, the individual contributions of various waste clearance deficits to ND progression remain largely unmapped. This complexity underscores the necessity for clinical researchers to adopt an integrative view, which is especially important when weighing the costs and benefits of novel treatment methods aimed at improving the efficiency of brain waste clearance.
IN VIVO IMAGING EVALUATION OF THE WASTE CLEARANCE SYSTEM
In this section, we provide a concise overview of in vivo imaging methods for assessing cerebral waste clearance. Since recent reviews have extensively introduced these techniques, our primary objective is to establish theoretical links between clearance mechanisms and their corresponding imaging biomarkers, while also offering practical recommendations on technological readiness to guide clinical researchers in methodology selection. Validated and practical methods are introduced in detail, while methods still under development are briefly mentioned.
DYNAMIC CONTRAST-ENHANCED MAGNETIC RESONANCE IMAGING
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) enables visualization of the spatiotemporal distribution of contrast agents. Its clinical applicability in human studies is increasingly being demonstrated[2,12-14]. Current implementations employ two distinct contrast administration protocols: intravenous injection[2] and intrathecal administration[14,15] of gadolinium-based contrast agents (GBCAs), each engaging fundamentally different clearance pathways.
Following intrathecal delivery, contrast agents ascend through the spinal canal into the subarachnoid space, subsequently entering brain tissue via perivascular spaces (PVS) before returning to the subarachnoid circulation or re-entering the vascular system[15]. Alternative clearance routes include direct efflux through perineural pathways or absorption by meningeal lymphatics. After leaving the cranial compartment, contrast agents may pass through the nasal mucosa and deep cervical lymph nodes. By contrast, with intravenous administration, contrast agents cross either the BBB or the blood-CSF barrier to enter the CSF and interstitial compartments[16,17], from where they are cleared through the same pathways described above.
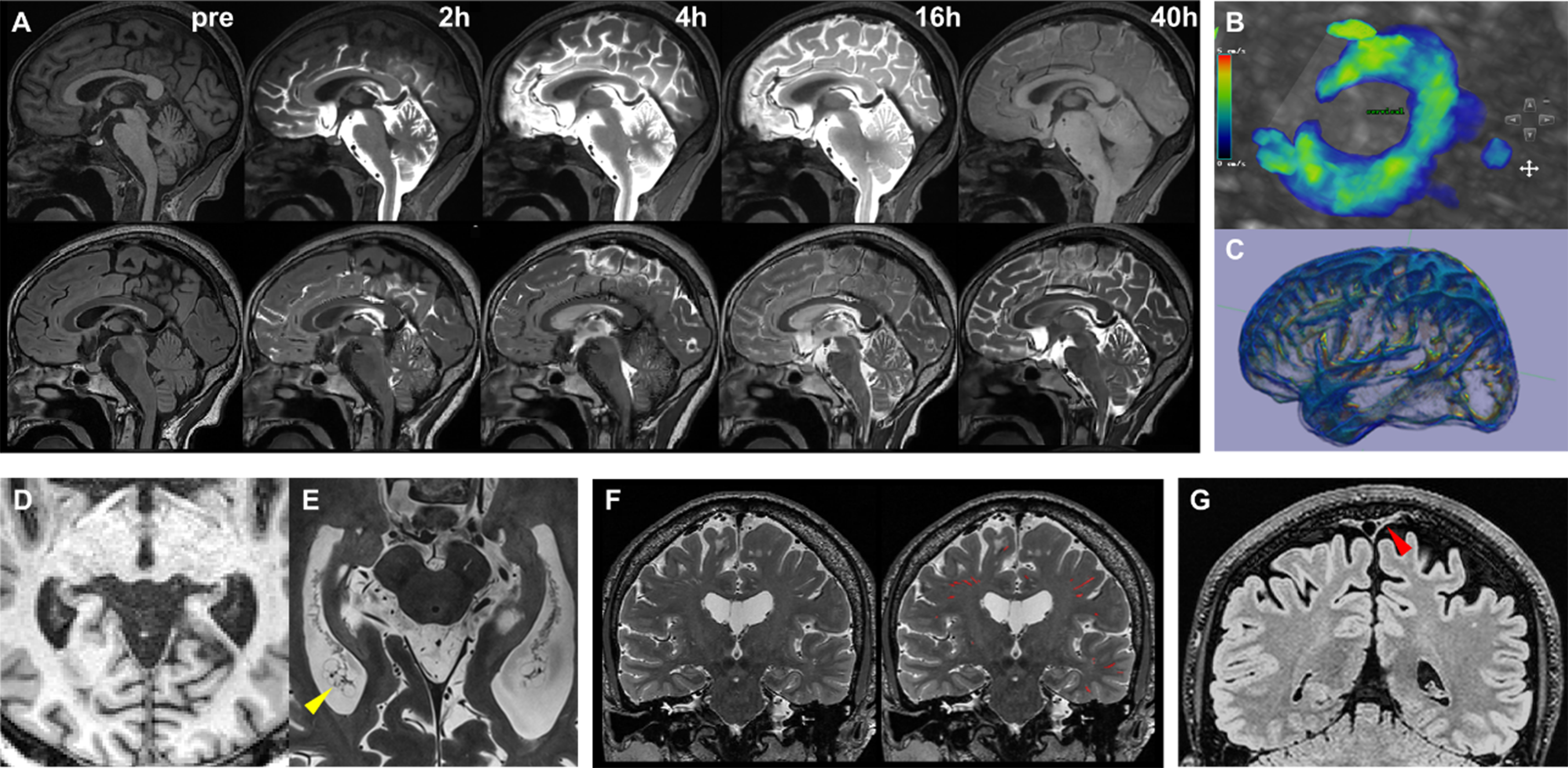
These distinct pathways result in unique spatiotemporal enhancement patterns. Intravenous protocols typically produce rapid enhancement (within tens of seconds) in ChP and meningeal lymphatics[2], followed by delayed signal increases in subarachnoid spaces and perivascular channels. With intrathecal administration, contrast agents reach the basal cisterns within 30 min, and then spread along periarterial spaces toward the convexity regions. Parenchymal and perivascular enhancement generally requires 1-2 days for full visualization [Figure 1][18], and complete clearance may take 3 days or longer. These mechanistic differences necessitate distinct clinical study designs: intravenous protocols typically involve rapid DCE-MRI acquisitions during initial contrast circulation (minutes post-injection), followed by delayed imaging at ~4 h to capture blood-to-CSF barrier dynamics. Conversely, intrathecal protocols require serial imaging at baseline, immediately post-injection (0-6 h), and at 12, 24, 48 h, and 7-day intervals to map the protracted cerebrospinal-lymphatic clearance trajectory.
Figure 1. (A) Following intrathecal contrast administration, the contrast agent ascends through the spinal canal into the basal cisterns and progressively distributes along the cerebral convexities. Pronounced parenchymal enhancement persists up to 40 h post-injection, as shown on axial T1-weighted (upper row) and FLAIR (lower row) sequences; (B) PC-MRI quantifies CSF flow dynamics at the foramen magnum level; (C)Low b-value diffusion-weighted imaging enables whole-brain mapping of CSF flow velocity; (D) Conventional 3 Tesla T1-weighted imaging delineates only the gross morphological features of the ChP; (E) Ultra-high-resolution 7 Tesla T1-weighted imaging reveals the intricate curled architecture, surface protrusions, and pathological cystic structures (yellow arrowhead) within the ChP; (F) High-resolution 3D T2-weighted imaging visualizes PVS, enabling neural network-based segmentation and quantitative analysis; (G) Optimized high-resolution FLAIR sequences provide clear visualization of PSD mater morphology (red arrowhead). PC-MRI: Phase-contrast magnetic resonance imaging; CSF: cerebrospinal fluid; ChP: choroid plexus; PVS: perivascular spaces; PSD: parasagittal dura.
It is crucial to note that while DCE-MRI is regarded as the reference method for in vivo assessment of cerebral waste clearance, the processes of tracer diffusion, trans-barrier transport, and elimination are influenced by numerous confounding factors. For example, posture and sleep-awake status may modulate CSF flow[11,19]. Therefore, these factors need to be controlled for or at least recorded and included as covariates in statistical analyses. In current DCE-MRI studies, parenchymal enhancement tends to be subtle and delayed, likely attributable to the low permeability of brain tissue to GBCAs given their large molecular size[20]. Moreover, different pathological proteins vary in molecular size and aggregation patterns, meaning the effects of glymphatic impairments may differ substantially across proteinopathies. This inherent physicochemical mismatch raises concerns about the validity of GBCA kinetics as a direct proxy for endogenous metabolic waste clearance. These methodological limitations underscore the need for cautious interpretation of DCE-MRI-derived clearance metrics. Furthermore, the clinical application of DCE-MRI remains constrained by its invasiveness and technical complexity, particularly with intrathecal protocols. Additional challenges include the absence of standardized imaging protocols and well-established diagnostic cut-off values for specific diseases.
FLOW AND DIFFUSION MRI
Cerebral waste clearance is fundamentally governed by fluid dynamics, which involve both the relatively rapid bulk flow of CSF through the ventricles and subarachnoid spaces, and the slower movement of glymphatic/interstitial fluid. MRI provides unparalleled capability to monitor water motion at both macroscopic and microscopic levels. Phase-contrast MRI (PC-MRI) excels at quantifying rapid bulk flow, while diffusion-weighted techniques are more sensitive to slower water diffusion.
PC-MRI
CSF flow velocities can reach several centimeters per second at anatomical bottlenecks such as the cerebral aqueduct, pontine cistern, and foramen magnum. This pulsatile flow is primarily driven by cerebral vascular compliance: CSF shifts cranially during arterial vasoconstriction and reverses during vasodilation. Standard PC-MRI implementations employ cardiac-gated acquisitions to resolve flow velocity variations across the cardiac cycle, enabling characterization of mean flow rates and waveform characteristics[21]. Notably, respiration can also drive CSF flow, but this factor has often been neglected in prior studies due to the technical challenges of multi-parametric gating. Spatial resolution trade-offs dictate protocol selection: 2D-PC-MRI provides superior in-plane resolution for unidirectional flow quantification (e.g., aqueductal flow), whereas 4D-PC-MRI enables assessment of multidirectional flow at the cost of lower resolution[22] and more complex post-processing.
Diffusion MRI
In the ventricles and subarachnoid spaces (excluding the aforementioned bottleneck regions), CSF flow velocities are substantially lower and therefore difficult to detect using PC-MRI, which suffers from a low signal-to-noise ratio for slow flows. Diffusion MRI offers superior sensitivity in these contexts. In diffusion imaging, CSF bulk flow can mimic diffusion-related signal decay. By applying low b-values (< 200 s/mm2), pseudo-diffusion coefficients can be estimated to reflect the velocity of slow CSF motion[23]. Cardiac-gated low b-value imaging (150 s/mm2) can reveal heartbeat-related CSF flow variations around major blood vessels[24], with flow directions consistent with theoretical assumptions[25]. Cerebrospinal fluid-based spatial statistics (CBSS) is a method that enables automated extraction of diffusion parameters from predefined ventricular and sulcal areas, facilitating population-level analysis[26]. Overall, low b-value diffusion imaging requires only modest scanner performance and acquisition parameters, making it clinically practical for neuroimaging studies. However, the extent to which CSF flow in ventricular and subarachnoid spaces influences glymphatic clearance efficiency remains unclear.
In PVS and interstitial compartments, where fluid velocities drop to micrometer-per-second scales, diffusion-based techniques offer greater sensitivity. The Diffusion Tensor Imaging Along the Perivascular Space (DTI-ALPS) method has gained increasing use[27]. The theoretical framework posits that near the upper level of the lateral ventricles, deep medullary veins run horizontally, while adjacent white matter bundles contain orthogonal fibers: vertically oriented projection fibers (Proj) and anteroposterior association fibers (Asso). Axial diffusivity (Dx) along the left-right axis reflects a combination of perivenous flow and radial fiber diffusion, whereas orthogonal diffusivities (Dy_proj and Dz_asso) capture pure radial diffusion. The ALPS index, calculated as (Dx_proj + Dx_asso)/(Dy_proj + Dz_asso), theoretically quantifies the excess diffusion attributable to perivascular flow.
Owing to its technical accessibility - requiring only conventional DTI acquisitions - and good test-retest reproducibility, the ALPS index has been widely adopted in clinical studies. Nonetheless, several limitations should be noted. First, the index can only be measured near the upper level of the lateral ventricles, as required by its theoretical framework. Whether this local measurement reliably reflects global glymphatic clearance efficiency remains debated. One study[28] reported a strong correlation between the ALPS index and tracer clearance measured by DCE-MRI, but the analysis did not account for potential confounders such as age, sex, or white matter degeneration. Second, multiple factors - including white matter degeneration, cerebral atrophy, and individual anatomical variations - can influence the orientation and morphology of major fiber tracts, complicating ROI placement and interpretation. These methodological limitations should be carefully considered in clinical applications.
STRUCTURAL MRI
Structural MRI is a critical tool for evaluating the anatomical components involved in cerebral waste clearance, including the ChP, PVS, and parasagittal dura (PSD). These structures undergo measurable morphological changes that may reflect their functional status in waste clearance.
The ChP, the primary site of CSF production, undergoes significant age-related and neurodegenerative alterations. Declines in CSF secretion correlate with structural degeneration, such as epithelial cell atrophy and stromal fibrosis[29]. While clinical MRI enables gross morphological visualization and volumetric quantification of the ChP[30], ultrahigh-field MRI can capture finer details, including its curled architecture, surface granular protrusions, and pathological cystic formations[31]. These structural features serve as indirect biomarkers for assessing secretory dysfunction and degenerative progression. PVS, functioning as the main conduits of the glymphatic clearance pathway, demonstrate dimensional and morphological variations that influence fluid dynamics. Enlarged PVS diameter or altered tortuosity may impair interstitial waste transport efficiency[32]. Three-dimensional, high-resolution T2-weighted imaging techniques enable precise visualization of PVS linear structures, while emerging open-source segmentation algorithms allow for automated quantification of their volume, spatial distribution, and morphological complexity[33]. The PSD, located adjacent to the superior sagittal sinus, has been identified as a key site for CSF efflux from the brain[14]. Signal characteristics in this region, potentially reflecting waste composition, can be delineated using optimized high-resolution FLAIR sequences[34].
Structural MRI is widely used in clinical practice due to its accessibility. With advances in deep learning, these anatomical structures can now be segmented automatically. The relationships between their morphological features and clinical function have been extensively studied. However, establishing a direct link between structural changes and specific physiological deficits remains challenging. For example, while increased ChP volume - a common finding across many diseases - has been observed, it remains unclear whether this reflects impaired CSF secretion. Similarly, it is not yet unknown whether alterations in PVS volume or shape influence CSF hydrodynamics within the PVS.
OTHER METHODS
In addition to the above-mentioned methodologies, many emerging techniques remain under development and validation. High-resolution diffusion MRI protocols have been experimentally applied to assess fluid dynamics within the PSD[35]. However, technical challenges persist due to the anatomical constraints of this narrow structure. Heavily T2-weighted 3D fast spin-echo imaging combined with improved multidirectional diffusion-sensitized driven-equilibrium (iMDDSDE) shows potential for quantifying low-velocity fluid movement through the thin PVS channels[36]. Intravoxel incoherent motion imaging has identified a distinct diffusion component, intermediate between capillary perfusion and parenchymal diffusion, which is believed to reflect perivascular fluid motion[37]. Chemical exchange saturation transfer MRI has demonstrated the ability to map metabolic waste concentrations in rodent brain parenchyma, but this method has not been tested in humans[38]. Dynamic PET imaging can evaluate tracer kinetics across different anatomical regions, providing insights into clearance pathways and rates[39,40]. Nevertheless, this approach carries radiation risks and is affected by confounding factors including nonspecific tracer binding and variable blood-brain/blood-CSF barrier integrity across pathological states.
IMAGING BRAIN WASTE CLEARANCE IN NDS
Amid rapid advancements in brain waste clearance theory and neuroimaging technologies, in vivo studies linking waste clearance dysfunction to aging and neurodegeneration have emerged as a key research frontier. Intrathecal contrast-enhanced DCE-MRI studies have revealed an age-dependent decline in clearance efficiency[12]. A landmark intravenous DCE-MRI study demonstrated impaired meningeal lymphatic drainage in PD patients, corroborated by animal models showing α-synucleinopathy-induced lymphatic damage[2]. However, the need for contrast agents has limited the broader application of DCE-MRI. Consequently, many studies have relied on retrospective analyses involving ChP volumetry, PVS characteristics, and DTI-ALPS indices. ChP hypertrophy has been consistently observed in AD patients[41,42], and mechanistically linked to neuroinflammation[43]. A recent 7T MRI study also reported a significantly higher prevalence of pathological ChP cysts in AD cohorts[31]. PVS enlargement exhibits distinct patterns across neurodegenerative disorders, with cortical PVS predominance in AD[44], contrasting with basal ganglia/midbrain PVS dilation in PD[45]. The DTI-ALPS index demonstrates broad clinical relevance, correlating strongly with pathological burden, clinical function, and disease deterioration in AD[46,47], PD[48], and frontotemporal dementia[49].
LIMITATIONS AND FUTURE PERSPECTIVES
While current neuroimaging studies have provided preliminary evidence linking impaired cerebral waste clearance to NDs, substantial knowledge gaps persist. The invasive nature of intrathecal contrast administration has precluded its application in patient cohorts, while intravenous contrast studies provide only a partial view of alterations in the meningeal-cervical lymphatic pathway. The relationships between structural parameters (e.g., ChP volume, PVS morphology) and CSF dynamics remain controversial[50], and the hierarchical contributions of specific clearance phases - CSF production, circulation, exchange, and drainage - to brain homeostasis and ND pathogenesis remain unresolved.
Emerging evidence suggests distinct clearance pathway alterations across ND subtypes. For instance, DCE-MRI studies demonstrate that PD patients exhibit significantly prolonged peak signal times in meningeal lymphatics compared to controls, whereas multiple system atrophy (MSA) and progressive supranuclear palsy (PSP) cohorts showed no significant differences[2]. AD is characterized by extensive pathological protein deposition in the ChP (potentially disrupting CSF secretion and circulation), a phenomenon less pronounced in PD[29,51]. These findings suggest that, although impaired clearance may contribute broadly to NDs, effective therapies will likely require disease-specific strategies. Recently, interventions such as cervical venous-lymphatic anastomosis and 40 Hz light stimulation[52] have been proposed to promote waste clearance, though their mechanisms and efficacy remain controversial. Imaging-based monitoring of clearance function may provide direct evidence to test their efficacy.
We advocate for a holistic framework that integrates multimodal imaging to investigate the diverse aspects of cerebral waste clearance pathophysiology. Imaging assessments could help identify abnormalities in specific pathways and subprocesses, guiding patient selection for targeted interventions. Furthermore, treatment effects should be evaluated with imaging to ascertain that the interventions truly improve the intended clearance function. Progress in this field will require close interdisciplinary collaboration bridging neuroimaging, basic research, and clinical neurology, ultimately enabling precision interventions tailored to ND-specific clearance deficits.
DECLARATIONS
Authors’ contributions
Made substantial contributions to the conception and writing of the paper: Huang P, Hong H, Zhang R
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the Natural Science Foundation of Zhejiang Province (No. LZ24H180002) and the National Natural Science Foundation of China (No. 82371907; No. 82101987).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370:50-6.
2. Ding XB, Wang XX, Xia DH, et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat Med. 2021;27:411-8.
3. Kaur J, Fahmy LM, Davoodi-Bojd E, et al. Waste clearance in the brain. Front Neuroanat. 2021;15:665803.
4. Wei H, Jiang H, Zhou Y, Xiao X, Zhou C, Ji X. Vascular endothelial cells: a fundamental approach for brain waste clearance. Brain. 2023;146:1299-315.
5. Aldea R, Weller RO, Wilcock DM, Carare RO, Richardson G. Cerebrovascular smooth muscle cells as the drivers of intramural periarterial drainage of the brain. Front Aging Neurosci. 2019;11:1.
6. Khasawneh AH, Garling RJ, Harris CA. Cerebrospinal fluid circulation: what do we know and how do we know it? Brain Circ. 2018;4:14-8.
7. Saunders NR, Dziegielewska KM, Fame RM, Lehtinen MK, Liddelow SA. The choroid plexus: a missing link in our understanding of brain development and function. Physiol Rev. 2023;103:919-56.
8. Bohr T, Hjorth PG, Holst SC, et al. The glymphatic system: current understanding and modeling. iScience. 2022;25:104987.
9. Klostranec JM, Vucevic D, Bhatia KD, et al. Current concepts in intracranial interstitial fluid transport and the glymphatic system: Part I - anatomy and physiology. Radiology. 2021;301:502-14.
10. Xie L, Zhang Y, Hong H, et al. Higher intracranial arterial pulsatility is associated with presumed imaging markers of the glymphatic system: an explorative study. Neuroimage. 2024;288:120524.
11. Fultz NE, Bonmassar G, Setsompop K, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 2019;366:628-31.
12. Zhou Y, Cai J, Zhang W, et al. Impairment of the glymphatic pathway and putative meningeal lymphatic vessels in the aging human. Ann Neurol. 2020;87:357-69.
13. Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain. 2017;140:2691-705.
14. Ringstad G, Eide PK. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat Commun. 2020;11:354.
15. van Osch MJP, Wåhlin A, Scheyhing P, et al. Human brain clearance imaging: pathways taken by magnetic resonance imaging contrast agents after administration in cerebrospinal fluid and blood. NMR Biomed. 2024;37:e5159.
16. Sun Y, Cao D, Pillai JJ, et al. Rapid imaging of intravenous gadolinium-based contrast agent (GBCA) entering ventricular cerebrospinal fluid (CSF) through the choroid plexus in healthy human subjects. Fluids Barriers CNS. 2024;21:72.
17. Naganawa S, Ito R, Kawai H, Taoka T, Yoshida T, Sone M. Confirmation of age-dependence in the leakage of contrast medium around the cortical veins into cerebrospinal fluid after intravenous administration of gadolinium-based contrast agent. Magn Reson Med Sci. 2020;19:375-81.
18. Yamamoto EA, Bagley JH, Geltzeiler M, et al. The perivascular space is a conduit for cerebrospinal fluid flow in humans: a proof-of-principle report. Proc Natl Acad Sci U S A. 2024;121:e2407246121.
19. Muccio M, Chu D, Minkoff L, et al. Upright versus supine MRI: effects of body position on craniocervical CSF flow. Fluids Barriers CNS. 2021;18:61.
20. Naganawa S, Taoka T, Ito R, Kawamura M. The glymphatic system in humans: investigations with magnetic resonance imaging. Invest Radiol. 2024;59:1-12.
21. Owashi KP, Liu P, Metanbou S, Capel C, Balédent O. Phase-contrast MRI analysis of cerebral blood and CSF flow dynamic interactions. Fluids Barriers CNS. 2024;21:88.
22. Vikner T, Johnson KM, Cadman RV, et al. CSF dynamics throughout the ventricular system using 4D flow MRI: associations to arterial pulsatility, ventricular volumes, and age. Fluids Barriers CNS. 2024;21:68.
23. Bito Y, Harada K, Ochi H, Kudo K. Low b-value diffusion tensor imaging for measuring pseudorandom flow of cerebrospinal fluid. Magn Reson Med. 2021;86:1369-82.
24. Wen Q, Tong Y, Zhou X, Dzemidzic M, Ho CY, Wu YC. Assessing pulsatile waveforms of paravascular cerebrospinal fluid dynamics within the glymphatic pathways using dynamic diffusion-weighted imaging (dDWI). Neuroimage. 2022;260:119464.
25. Han G, Jiao B, Zhang Y, et al. Arterial pulsation dependence of perivascular cerebrospinal fluid flow measured by dynamic diffusion tensor imaging in the human brain. Neuroimage. 2024;297:120653.
26. Chen Y, Hong H, Nazeri A, Markus HS, Luo X. Cerebrospinal fluid-based spatial statistics: towards quantitative analysis of cerebrospinal fluid pseudodiffusivity. Fluids Barriers CNS. 2024;21:59.
27. Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol. 2017;35:172-8.
28. Zhang W, Zhou Y, Wang J, et al. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage. 2021;238:118257.
29. Delvenne A, Vandendriessche C, Gobom J, et al. Involvement of the choroid plexus in Alzheimer’s disease pathophysiology: findings from mouse and human proteomic studies. Fluids Barriers CNS. 2024;21:58.
30. Li J, Hu Y, Xu Y, et al; Alzheimer’s Disease Neuroimaging Initiative. Associations between the choroid plexus and tau in Alzheimer’s disease using an active learning segmentation pipeline. Fluids Barriers CNS. 2024;21:56.
31. Zhen Z, Zhang R, Gui L, et al. Choroid plexus cysts on 7T MRI: relationship to aging and neurodegenerative diseases. Alzheimers Dement. 2025;21:e14484.
32. Wang S, Huang P, Zhang R, et al. Quantity and morphology of perivascular spaces: associations with vascular risk factors and cerebral small vessel disease. J Magn Reson Imaging. 2021;54:1326-36.
33. Huang P, Liu L, Zhang Y, et al; Alzheimer’s Disease Neuroimaging Initiative. Development and validation of a perivascular space segmentation method in multi-center datasets. Neuroimage. 2024;298:120803.
34. Albayram MS, Smith G, Tufan F, et al. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat Commun. 2022;13:203.
35. Liu YJ, Lin SC, Liao CH, et al. MUSE and PROPELLER DWI for ADC in parasagittal dura: insights from high-resolution and reduced-distortion DWI. Sci Rep. 2025;15:7473.
36. Ran L, He Y, Zhu J, et al. Characterizing cerebrospinal fluid mobility using heavily T2-weighted 3D fast spin echo (FSE) imaging with improved multi-directional diffusion-sensitized driven-equilibrium (iMDDSDE) preparation. J Cereb Blood Flow Metab. 2024;44:105-17.
37. Wong SM, Backes WH, Drenthen GS, et al. Spectral diffusion analysis of intravoxel incoherent motion MRI in cerebral small vessel disease. J Magn Reson Imaging. 2020;51:1170-80.
38. Chen Y, Dai Z, Fan R, et al. Glymphatic system visualized by chemical-exchange-saturation-transfer magnetic resonance imaging. ACS Chem Neurosci. 2020;11:1978-84.
39. de Leon MJ, Li Y, Okamura N, et al. Cerebrospinal fluid clearance in Alzheimer disease measured with dynamic PET. J Nucl Med. 2017;58:1471-6.
40. Li Y, Rusinek H, Butler T, et al. Decreased CSF clearance and increased brain amyloid in Alzheimer’s disease. Fluids Barriers CNS. 2022;19:21.
41. Jeong SH, Park CJ, Cha J, et al. Choroid plexus volume, amyloid burden, and cognition in the Alzheimer’s disease continuum. Aging Dis. 2024;16:552-64.
42. Jiang J, Zhuo Z, Wang A, et al. Choroid plexus volume as a novel candidate neuroimaging marker of the Alzheimer’s continuum. Alzheimers Res Ther. 2024;16:149.
43. Xu H, Lotfy P, Gelb S, et al. The choroid plexus synergizes with immune cells during neuroinflammation. Cell. 2024;187:4946-63.e17.
44. Banerjee G, Kim HJ, Fox Z, et al. MRI-visible perivascular space location is associated with Alzheimer’s disease independently of amyloid burden. Brain. 2017;140:1107-16.
45. Li Y, Zhu Z, Chen J, Zhang M, Yang Y, Huang P. Dilated perivascular space in the midbrain may reflect dopamine neuronal degeneration in Parkinson’s disease. Front Aging Neurosci. 2020;12:161.
46. Huang SY, Zhang YR, Guo Y, et al; Alzheimer’s Disease Neuroimaging Initiative. Glymphatic system dysfunction predicts amyloid deposition, neurodegeneration, and clinical progression in Alzheimer’s disease. Alzheimers Dement. 2024;20:3251-69.
47. Hong H, Hong L, Luo X, et al; Alzheimer’s Disease Neuroimaging Initiative (ADNI). The relationship between amyloid pathology, cerebral small vessel disease, glymphatic dysfunction, and cognition: a study based on Alzheimer’s disease continuum participants. Alzheimers Res Ther. 2024;16:43.
48. Zhou C, Jiang X, Guan X, et al. Glymphatic system dysfunction and risk of clinical milestones in patients with Parkinson disease. Eur J Neurol. 2024;31:e16521.
49. Jiang D, Liu L, Kong Y, et al; Frontotemporal Lobar Degeneration Neuroimaging Initiative. Regional glymphatic abnormality in behavioral variant frontotemporal dementia. Ann Neurol. 2023;94:442-56.
50. Thomas JH. Fluid dynamics of cerebrospinal fluid flow in perivascular spaces. J R Soc Interface. 2019;16:20190572.
51. Tadayon E, Pascual-Leone A, Press D, Santarnecchi E; Alzheimer’s Disease Neuroimaging Initiative. Choroid plexus volume is associated with levels of CSF proteins: relevance for Alzheimer’s and Parkinson’s disease. Neurobiol Aging. 2020;89:108-17.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.