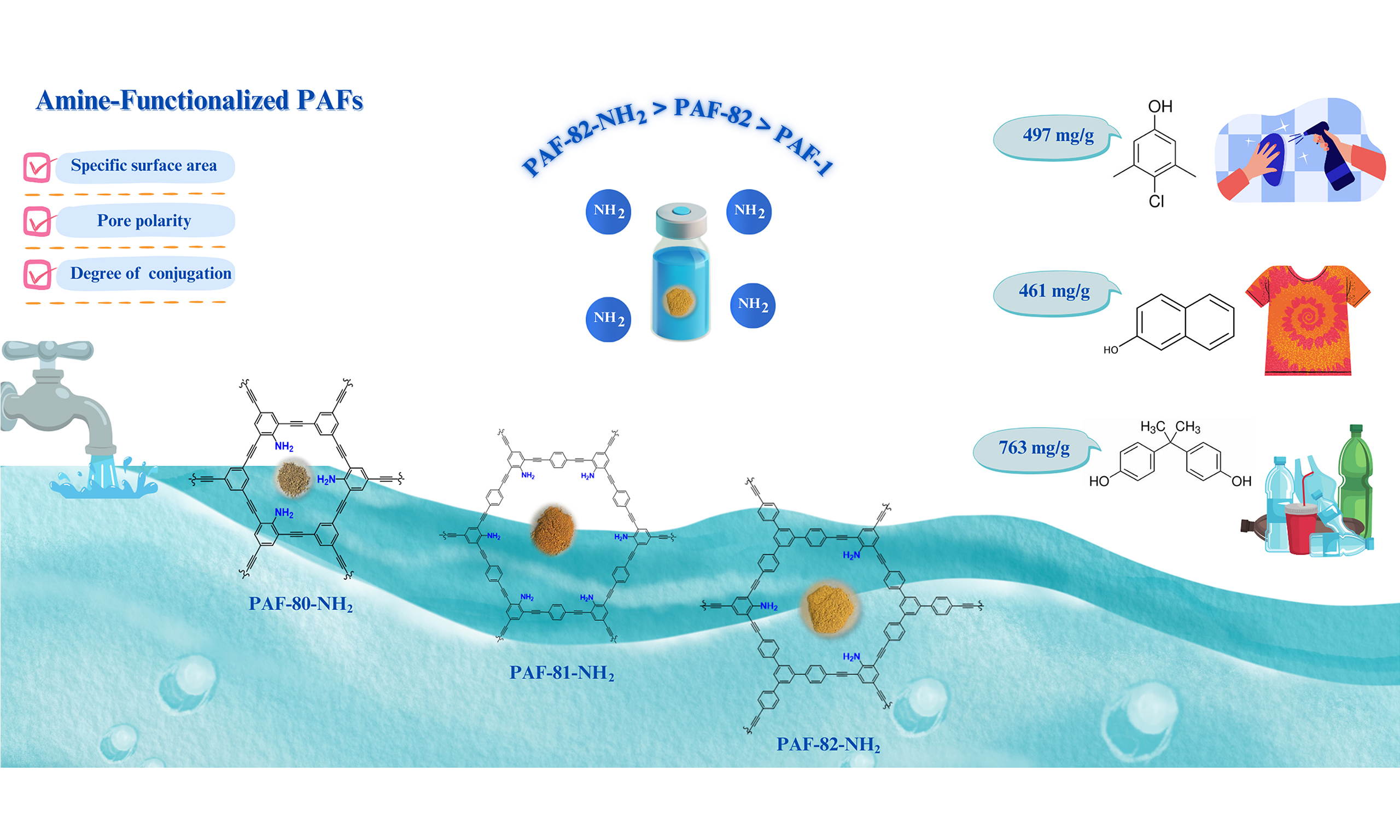
Amine-functionalized porous aromatic frameworks for efficient adsorption of hazardous organic micropollutants in water
Abstract
Amongst the most hazardous organic micropollutants (OMPs), β-naphthol, para-chloro-meta-xylenol, and bisphenol-A are highlighted due to their toxicity and persistent nature in aquatic environments. This work presents a series of cost-effective novel amine-functionalized porous aromatic frameworks (PAFs) for the adsorption of OMPs: PAF-82-NH2, PAF-81-NH2, and PAF-80-NH2. PAF-82-NH2 shows outstanding adsorption performance by exhibiting the highest Brunauer-Emmett-Teller surface area, polarity match with pollutants, and structural conjugation, with no loss in removal efficiency after five cycles of regeneration. The Langmuir adsorption capacity of PAF-82-NH2 for three OMPs outstrips the most previously reported adsorbents: 461 mg·g-1 for β-naphthol,
Keywords
INTRODUCTION
Although water is considered the most abundant resource on Earth, it remains unavailable for consumption due to various micropollutants present at trace yet toxic concentrations, which make water treatment techniques challenging globally[1]. Organic micropollutants (OMPs) are extensively located in water resources as anthropogenic substances, including pesticides, pharmaceutically active compounds (phACs), synthetic resins, and personal care products (PCPs). In recent years, OMPs have garnered significant attention as an environmental issue, raising concerns about human health due to their low concentrations, toxicity, and persistent nature in aquatic environments[2,3]. Some health hazards associated with OMPs include bisphenol-A (BPA, found in polycarbonate plastics), which is related to endocrine disruption[4], and β-naphthol (2-NO, a dye intermediate), which is responsible for respiratory irritations and asthma[5]. The most frequently used disinfectant, para-chloro-meta-xylenol (PCMX), poses serious risks to aquatic life due to its stability in the marine environment[6]. To thoroughly understand the OMPs in question, we summarize their physicochemical characteristics in Table 1, which provides essential information about three OMPs: 2-NO, PCMX, and BPA. The three hazardous OMPs listed are common in everyday products, allowing for their rapid introduction into various water systems. Existing water treatment processes lack high removal efficiencies for these OMPs[18]. Therefore, different methods have been introduced for their removal, including advanced oxidation processes (AOPs)[19], biodegradation[20], photocatalytic degradation[21], and adsorption[8]. Among these, adsorption is the most reliable technique, providing operational ease and low energy consumption for removing OMPs from drinking water and industrial effluents[22,23].
Summary of the physicochemical characteristics of the three OMPs: BPA, 2-NO, and PCMX
| Characteristics | BPA | 2-NO | PCMX |
| Chemical formula | C15H16O2 | C10H8O | C8H9ClO |
| Usage | Polycarbonate-plastics | Dye intermediate | Antiseptic |
| Water solubility (mg·L-1) | 120-300[7] | 740[8] | 300[9] |
| Pka | 10.3[7] | 9.6[8] | 9.76[10] |
| LogKow | 2.20-3.82[11] | 2.7[8] | 3.27[12] |
| Limits of detection (µg·L-1) | 200[13] | 0.19[8] | 0.2545[14] |
| Concentration in WW* (µg·L-1) | 0.016-1.465[15] | 5-30[16] | 0.06-10.6[17] |
Focusing on the efficiency of the adsorption technique, several solid adsorbents have been developed and employed for the adsorption of toxic OMPs from water resources, for instance, activated carbon[24], clay minerals, high silica zeolites[25], carbon nanotubes[7], metal-organic frameworks (MOFs)[8] and covalent organic frameworks (COFs)[26]. Due to the crystallinity of structure and tunable porosity, COFs have demonstrated outstanding potential for capturing water contaminants such as heavy metals and dyes[27]. Moreover, functional groups such as sulfonic acid and amine in COFs facilitate chemical interactions with water contaminants through hydrogen bonding, ionic bonding, and dipole interactions. However, COFs suffer from structural instability, which limits their industrial applicability, especially in harsh environments[28]. Conversely, MOFs exhibit significant adsorption performance for heavy metals, gases, and organic pollutants. However, their sensitivity to acidic or basic conditions causes structural degradation, reducing their useability[29]. Along with limited adsorption capacities, these solid adsorbents also lack fast uptake, hydrolytic stability, and good reproducibility. Research has focused on developing porous materials that serve fast adsorption, significant hydrolytic stability, and exceptional recyclability to address these shortcomings. Because of the large surface area and strong chemical and thermal stability, porous aromatic frameworks (PAFs) can be the finest candidates for eliminating OMPs from water[22]. COFs and PAFs are two different classes of porous materials. Both materials can be synthesized by organic/aromatic building units and employed in valuable applications such as gas storage, catalysis, and adsorption. However, if these materials are carefully observed, there are fundamental differences in structure and synthesis. PAFs are emerging amorphous materials formed through irreversible coupling reactions (e.g., Suzuki or Yamamoto coupling), resulting in highly stable, fully three-dimensional porous networks with tunable porosity. In contrast, COFs are crystalline frameworks formed via reversible reactions, such as Schiff base or boronic acid condensations, allowing self-correction and long-range order. Unlike COFs, PAFs are not a subclass but a separate family of materials with unique structural characteristics, including isotropic porosity and exceptional stability[30]. Due to irreversible covalent bonding, PAFs are significantly more stable than COFs. Therefore, a robust framework allows PAFs to showcase excellent stability under harsh conditions, which is absent in COFs. PAFs undergo strategic synthesis with rigid building units in their framework, carrying predesigned geometries. For the porosity of PAFs, framework interpenetration, building unit’s geometries, and mechanism of synthetic reactions are crucial factors. PAFs can be categorized based on their pore size as microporous (< 2 nm) to mesoporous (2-50 nm). Their framework backbone is stabilized by C–C covalent bonding. PAFs contain a high density of aromatic motifs in their frameworks, leading to better π-π interactions with aromatic systems of encountering hazardous OMPs with high uptake capacities for incoming aromatic systems[31]. As far as the chemistry of OMPs is concerned, it has been seen that they all contain distinct polar functional groups in their structure, for instance, hydroxyl group (-OH), amine group (-NH2), and carboxylic group (-COOH). To remove OMPs by adsorption techniques, interactions must be formed with polar pollutants and PAFs. This can be possible by hydrogen bonding as supramolecular interactions by developing polarity within walls of porous material[22].
Recently, PAFs have been under much attention as emerging porous materials with rigid frameworks, high surface area, and exceptional stability, carrying advanced applications. Most PAFs are hydrophobic and constructed by C–C bonds among aromatic units. Therefore, the polarity engineering strategy effectively enhances channel polarity and surface wettability for efficient adsorption and removal of polar OMPs from aquatic environments. Indulging suitable polar functional groups to have a good polarity match between guest pollutants and channel walls of PAF, specific water containments can be easily located and removed. For water purification from OMPs by PAFs, polarity at pore channels and surface wettability is crucial[32]. It has been reported that amino group modifications positively influence the adsorption of organic substances from water, along with enhanced adsorption capacities[33].
Focusing on all the points mentioned earlier, we are introducing a series of cost-effective amine-functionalized PAFs (PAF-82-NH2, PAF-81-NH2, and PAF-80-NH2) from the reaction of a single amine group-based motif, 2,4,6-tribromoaniline, along with aromatic ethynyls building units, the same as used for PAF-80, PAF-81, and PAF-82[22]. This is to test the adsorption performance when they come in contact with OMPs in water. The 2,4,6-tribromoaniline monomer has been selected to adopt cost-effective novel PAF synthesis, and through this monomer, the amine group can quickly be introduced in the PAF framework. Ethynyl building units offer unique sites for binding and chemical stability along with aromatic motifs carrying a high density of π-electrons, which is a significant factor for micropollutant adsorption.
Attaching amine group (-NH2) on the hydrophobic backbone of PAFs can develop polarity within channel walls, and consequently, interactions between PAF channels and OMPs via hydrogen bonding can be significantly improved. Considering this, we take advantage of the π-electronic system carried by aromatic rings of ethynyl groups and the potential of the amine group to enhance the uptake capacity of toxic OMPs from water.
Our research is centered on exploring the potential of amine (-NH2)-functionalized PAFs in enhancing the adsorption capacity of hazardous OMPs. As novel amine-functionalized PAFs utilizing three aromatic ethynyls building units as used for PAF-82, PAF-81, and PAF-80, with the replacement of the hydroxyl
EXPERIMENTAL
Chemicals and characterization
Chemical drugs and anhydrous solvents used in the synthetic chemical reactions were bought from suppliers and consumed just as supplied, requiring no additional purification. Materials
Synthesis of PAF-82-NH2, PAF-81-NH2, and PAF-80-NH2
Sonogashira-Hagihara cross-coupling chemical reaction catalyzed by tetrakis(triphenyl-phosphine) palladium was employed for the synthesis of amine-functionalized novel PAF-82-NH2, PAF-81-NH2, and PAF-80-NH2.
PAF-82-NH2
For the synthesis of PAF-82-NH2, 2,4,6-tribromoaniline (99 mg, 0.3 mmol), 1,3,5-tris(4-ethynyl phenyl)benzene (113.5 mg, 0.3 mmol), tetrakis(triphenyl-phosphine) palladium (228.5 mg) and copper(I) iodide (7.5 mg) were added into a round bottom reaction flask with two necks (volume of 100 ml), having extra dry DMF and triethylamine (1:1 v/v) 15 mL of a mixture of two solvents. When the reaction mixture was ready in a round bottom flask with reactants and solvent, degassing by three freeze-pump-thaw cycles was applied. Then, this reaction mixture is left in the N2 environment under heating at 100 °C for almost
PAF-81-NH2
Similarly, brownish-red PAF-81-NH2 was synthesized by reacting 2,4,6-tribromoaniline (99 mg, 0.3 mmol) and 1,4-Diethynylbenzene (113.5 mg, 0.45 mmol) in the presence of the same concentration of catalysts as mentioned above, tetrakis(triphenyl-phosphine) palladium (228.5 mg) and copper(I) iodide (7.5 mg), following rest of the procedure without any change as adopt for PAF-82-NH2.
PAF-80-NH2
PAF-80-NH2 was obtained by taking 2,4,6-tribromoaniline (99 mg, 0.3 mmol) and 1,3,5-triethynylbenzene (45 mg, 0.3 mmol) as a brown powder. The concentration of catalysts and the rest of the process is the same as for PAF-82-NH2.
Mole ratios and obtained yields for three amine-functionalized PAF materials are summarized in Supplementary Table 1. The synthetic route representation is given in Figure 1.
PAF-1 synthesis
PAF-1 is synthesized according to the reported method[34], which is given in the Supplementary Section 1.
Adsorption experiments
Adsorption kinetics
Three hazardous OMPs, i.e., 2-NO, PCMX, and BPA, exclusively present in water resources, were selected for adsorption kinetic studies at room temperature (298 K). The removal performance of amine-functionalized novel PAFs was analyzed based on the adsorption of these OMPs from water while treated at low concentrations. To perform kinetic investigations, 15 mL of pollutants (2-NO, PCMX, and BPA) solutions were taken from stock solution (0.1 mmol·L-1) and mixed uniformly with a stirring magnet after adding 5 mg of each adsorbent. After mixing OMPs with amine-functionalized PAFs for some time of adsorption, a specific volume is drawn out with the help of a syringe.
A 0.22 mm disposable hydrophilic nylon syringe filter filtrated drawn aliquots. The concentration of each pollutant in solution form, initially (0.1 mmol·L-1) and after filtration, was calculated through a UV-vis spectrophotometer with the help of a standard curve at lambda max (λmax) for each pollutant (i.e., 2-NO at 274 nm, PCMX at 267 nm, and BPA at 276 nm). To provide more precise data, adsorption kinetic fitting was performed using an average of three concordant measurements. These three concordant measurements were further used to calculate the standard deviation in adsorption data. All adsorption experiments for the kinetic study of amine-functionalized PAFs were conducted under similar conditions with 0.33 mg·mL-1 pollutant concentration. This concentration is supposed to mimic highly pollutant aquatic environments to evaluate real-time removal efficiencies. This enables us to ensure sufficient saturation of the adsorptive sites within a measurable timeframe, allowing us to accurately determine the time needed for the materials to reach equilibrium.
After treating micro-pollutants (2-NO, PCMX, and BPA) with each adsorbent (PAF-82-NH2, PAF-81-NH2, and PAF-80-NH2), the removal efficiency was evaluated according to
Where Co (mmol·L-1) and Ct (mmol·L-1) represent the concentrations of pollutants at t = 0 initially and after some specific time t = t treated with PAFs, respectively.
Adsorption Capacity of adsorbents towards these pollutants was measured according to
Where qt (mg·g-1) represents the pollutant amount absorbed by 1 g of absorbent after being treated for a specific time t = t (min), and Co (mmol·L-1) and Ct (mmol·L-1) are the preliminary and remaining concentrations of pollutant at t = 0 and t = t, respectively. Mw is the molar mass of the pollutant being treated for removal, V (L) is the pollutant solution volume, and m (g) is the mass of adsorbent added in the pollutant solution for adsorption.
The pseudo-first-order adsorption model of Lagergren[35] interprets the adsorption rate of the adsorbent, represented by
Where qe represents the pollutant’s adsorbed amount by the adsorbent (mg·g-1) at equilibrium. In contrast, qt is the adsorbed pollutant amount (mg·g-1) at a specific time t = t (min), and K1 is the first-order rate constant with units g·mg-1·min-1.
Adsorption isotherms
Solutions of 2-NO, PCMX, and BPA with concentrations varying from various points, mimicking OMPs in water in low concentration (0.1 to 1.0 mmol), were made for this investigation. Each adsorbent (PAF-82-NH2, PAF-81-NH2, PAF-80-NH2, and PAF-1) was added in an amount of 5 mg into 15 mL of pollutant solution from variable concentrations in glass vials. Then, all mixtures were stirred at a constant speed for 24 h so that all PAFs would become saturated with pollutants through adsorption. After 24 h of stirring, treated samples were filtered with the help of a 0.22 mm hydrophilic filter membrane, and the resultant adsorption solutions were analyzed by a UV-vis spectrophotometer. The Langmuir adsorption model illustrates adsorption isotherm fitting[36] according to
Where qe (mg·g-1) represents the specific amount of pollutant being adsorbed at equilibrium, and qmax,e
Restoration of amine-functionalized PAFs
For the sake of regeneration, amine-functionalized PAFs, after being treated with pollutants, were soaked in ethanol (EtOH) for a time interval of almost 3 h to allow the desorption of the tested adsorbed pollutants, thus enabling the easy regeneration of the PAF framework. After desorption with ethanol, these polymers were wholly dried and reused for pollutant adsorption. This whole procedure is repeated five times to evaluate amine-functionalized PAF recyclability.
RESULTS AND DISCUSSION
Considering the chemical synthetic route depicted in Figure 1, PAF-82-NH2, PAF-81-NH2, and PAF-80-NH2 contain a framework incorporating three different building units. These building units typically react with a single amine-bearing monomer through the Sonogashira-Hagihara cross-coupling reaction. FTIR spectroscopy primarily comprehends the evidence of reaction completion. Carefully analyzing the FTIR peaks of novel amine-functionalized PAFs and their building units, the stretching vibrations of an alkynyl group at the terminal and C-Br portion in the interacting monomers are shown by the strong bands at 3,298 and 1,064 cm-1 [Supplementary Figure 1].
The successful synthesis of amine-functionalized PAFs is linked to the disappearance of the C–H bond in the terminal alkyne group as it breaks, along with the rupture of the C–Br bond. These bonds break along the synthesis of amine-functionalized PAFs, thus representing the designed cross-coupling reaction completed between monomers. Additionally, amine-functionalized PAFs and 2,4,6-tribromoaniline showed intense bands at 3,420 cm-1. This confirms that amine group -NH2 remains untacked during this coupling reaction. Other than these significant peaks, some low-intensity bands appear at around 1,800-2,200 cm-1 for each synthesized PAF, associated with the stretching vibration mode of C≡C alkyne moieties. From the FTIR spectra of PAF-1, its synthesis is confirmed by the absence of a C-Br peak and the presence of =C–H (2,842 cm-1) and C=C (1,370 cm-1) peaks, as shown in Supplementary Figure 2A. TGA was performed to investigate how PAF-82-NH2, PAF-81-NH2, and PAF-80-NH2 are stable thermally
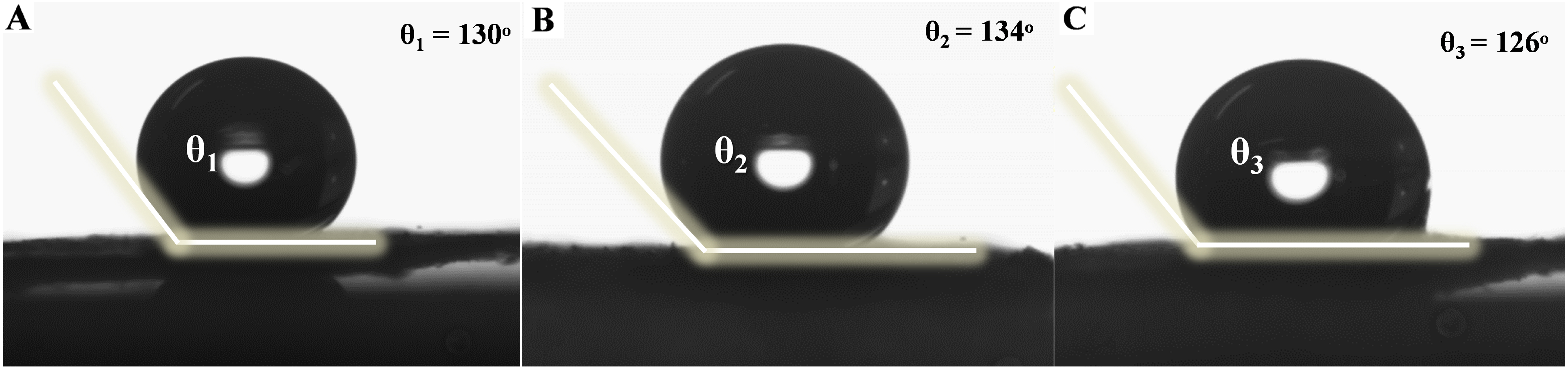
Figure 2. Contact angle of amine-functionalized PAFs: (A) PAF-80-NH2; (B) PAF-81-NH2; (C) PAF-82-NH2. PAFs: Porous aromatic frameworks.
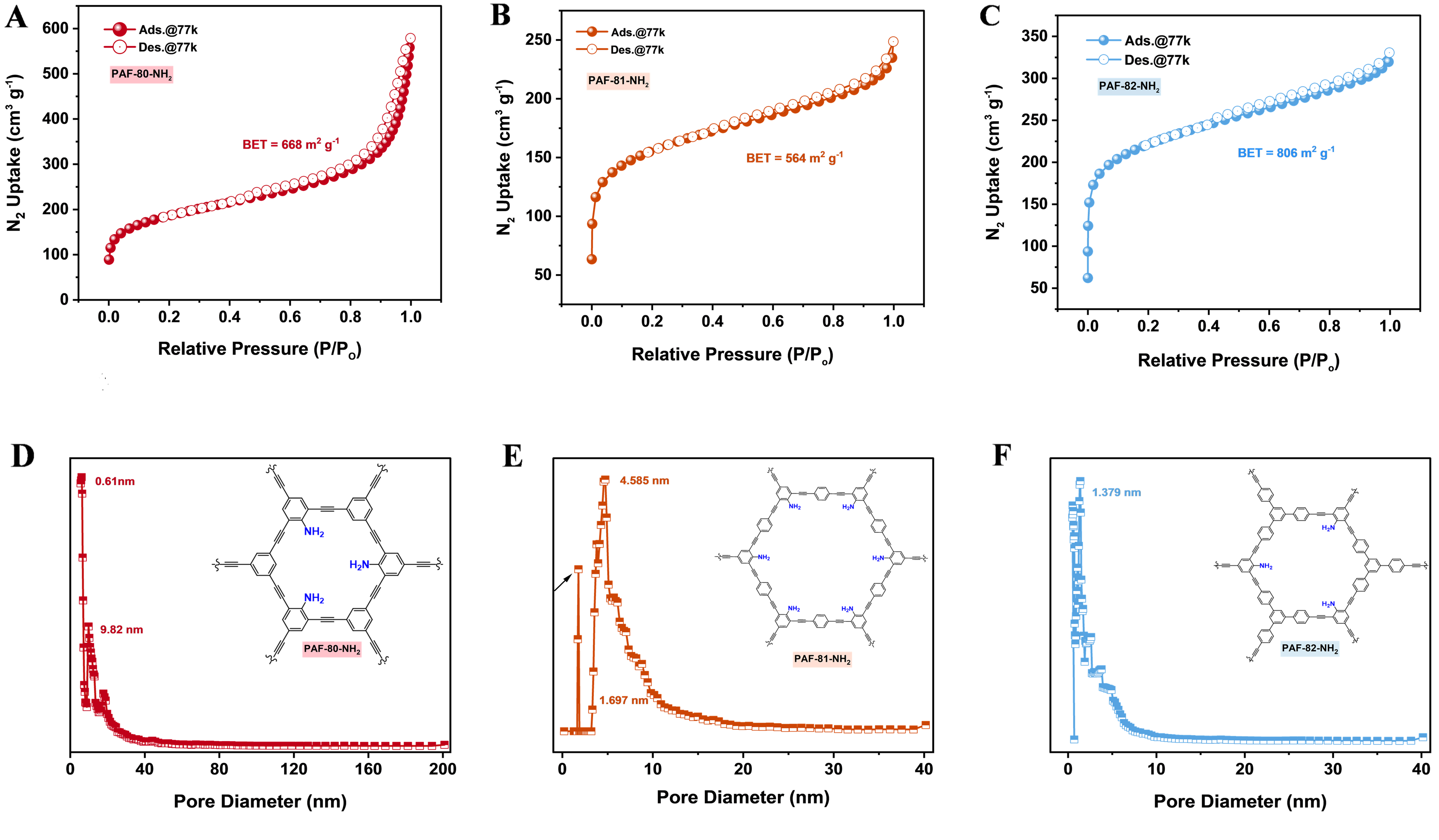
These results showed that amine-functionalized PAFs possess almost similar surface wettability because of very little difference in contact angles. These PAFs still have hydrophobic surfaces just because of excessive ethynyl aromatic units, irrespective of amine groups, as amine groups participated in polarity development at pore channels of the porous framework. For the understanding of the textural properties of amine-functionalized PAFs, N2 adsorption-desorption analysis was conducted at 77 K. Before this analysis, three amine-functionalized PAF materials were activated by heating at 100 °C for 12 h to remove those guest molecules, which could get trapped in the framework’s channels. The N2 sorption isotherm of PAF-80-NH2 comes under the type I adsorption curve category by considering Figure 3, showing a strong ability to absorb nitrogen at low relative pressure linked to the microporous properties [Figure 3A]. There is no typical type I isotherm provided by PAF-81-NH2. Nonetheless, the high-pressure zone exhibits a steady linear increase, as indicated in Figure 3B. Similarly, the N2 sorption isotherm of PAF-82-NH2 does not come under traditional isotherm type I; PAF-82-NH2 showed a more gradual linear increase in the high-pressure zone as compared to PAF-81-NH2 as shown in Figure 3C. The BET model was employed to calculate the surface areas of amine-functionalized PAF materials. The resultant BET of PAF-82-NH2, PAF-81-NH2, and PAF-80-NH2 is 806, 564, and 668 m2·g-1, respectively [Figure 3A-C]. Increasing order of BET surface area among amine-functionalized PAFs (m2·g-1) is PAF-82-NH2 > PAF-80-NH2 > PAF-81-NH2. Following the same procedure, PAF-1 had a BET of 5,600 m2·g-1, as in the literature [Supplementary Figure 2B]. In addition to BET, PSD of amine-functionalized PAF materials was also analyzed by considering NLDFT. Furthermore, the sizes of PAF-82-NH2, PAF-81-NH2, and PAF-80-NH2 pores are typically centered at 1.379, 1.697/4.585, and 0.61/9.82 nm, respectively [Figure 3D-F], according to analyzed data.
Figure 3. (A-C) N2 adsorption-desorption isotherms; (A) N2 adsorption-desorption isotherms of PAF-80-NH2; (B) N2 adsorption-desorption isotherms of PAF-81-NH2; (C) N2 adsorption-desorption isotherms of PAF-82-NH2; (D-F) PSD using NLDFT model; (D) PSD of PAF-80-NH2; (E) PSD of PAF-81-NH2; (F) PSD of PAF-82-NH2. PAF: Porous aromatic framework; PSD: pore size distribution; NLDFT: nonlocal density functional theory.
When amine-functionalized PAFs are constructed successfully, adsorption experiments are conducted for the evaluation of the removal capacities of these porous materials towards hazardous OMPs from water. For this purpose, OMPs from our daily lives were selected, which are abundantly consumed, difficult to degrade, and dangerous to human health and the environment. The three different phenolic pollutants, 2-NO, PCMX, and BPA, from the dye industry, PCPs sector, and plastic industry are associated with nervous system disruption, endocrine disruption, and respiratory tract diseases in the human body upon exposure, even at low concentrations[38]. These selected three OMPs served as model containments to run checks upon amine-functionalized PAF materials. For adsorption experiments, all maintained conditions were kept similar. For the determination of the concentration of OMPs, maximum absorption was observed at wavelengths 267, 274, and 276 nm for 2-NO, PCMX, and BPA aqueous solutions, respectively.
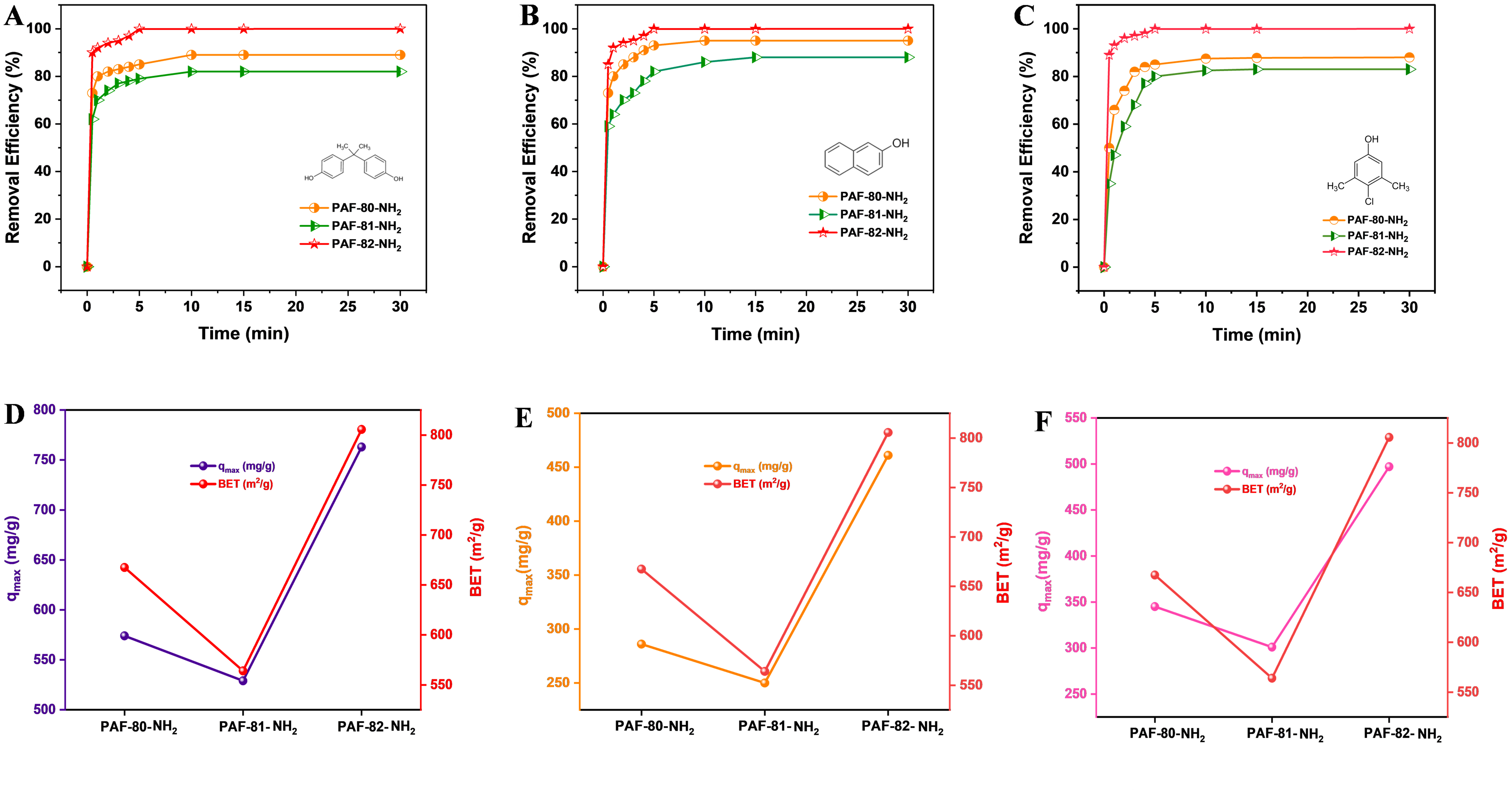
The removal performance of amine-functionalized PAF materials was found by using UV-vis spectra based on the removal of model pollutants from their aqueous solutions as a function of time. We got different adsorption responses from various amine-functionalized PAFs. Saturated adsorption time and rate are important factors concerning the elimination efficacy of these PAFs. The kinetic adsorption curves of PCMX, 2-NO, and BPA onto PAF-82-NH2, PAF-81-NH2, and PAF-80-NH2 are given in Figure 4. In the first 30 s of treatment, elimination efficiency of PAF-82-NH2 against BPA was 90%. Later, equilibrium was attained after 15 min, as shown in Figure 4A. After equilibrium was successfully achieved, the removal rate was 100%. In contrast, PAF-80-NH2 and PAF-81-NH2 showed removal efficiencies of 73% and 62% in the starting 30 s of stirring, needing 20 and 30 min to attain adsorption equilibrium.
Figure 4. (A-C) Kinetic adsorption curves; (A) Adsorption curve of 0.1 mM BPA; (B) Adsorption curve of 0.1 mM 2-NO; (C) Adsorption curve of 0.1 mM PCMX by 0.33 mg·mL-1 amine-functionalized PAFs over specific time intervals; (D-F) Relationship between BET of amine-functionalized PAFs and the adsorption capacity of three micropollutants; (D) Adsorption of BPA; (E) Adsorption of 2-NO; (F) Adsorption of PCMX. BPA: Bisphenol-A; PCMX: para-chloro-meta-xylenol; PAFs: porous aromatic frameworks; BET: Brunauer-Emmett-Teller.
Regarding the removal performance of amine-functionalized PAF materials for the second pollutant, PAF-82-NH2 removal efficiency towards 2-NO reached 85% within the initial 30 s and 20 min required to attain an equilibrium state. Then, removal efficiency reached 100%, higher than that of PAF-80-NH2 and PAF-81-NH2, as shown in Figure 4B. Similarly, it was found that the elimination efficiency of PAF-82-NH2 towards PCMX reached 89% within 30 s, and after that, 10 min were required to achieve equilibrium. After equilibrium, the removal efficiency was 100%. PAF-80-NH2 and PAF-81-NH2 showed only 50% and 35% removal within 30 s, requiring 25 and 30 min to independently attain adsorption equilibrium, as in Figure 4C. However, once adsorption equilibrium was established, PAF-80-NH2 and PAF-81-NH2 showed higher than 80% removal of all three model pollutants.
Amine-functionalized PAF materials can efficiently remove traces of OMPs from water. Around 10-15 min are required to get fast adsorption rates of these amine-functionalized PAF materials because of the availability of many adsorption sites initially when adsorption just started. Amine functional groups on PAF channel walls locate phenolic pollutants first, and then, as pollutants occupy adsorption sites, the adsorption rate slows down until equilibrium is achieved. According to the kinetic adsorption curves, the increasing order of removal efficiency of amine-functionalized PAF materials for model OMPs is PAF-82-NH2 > PAF-80-NH2 > PAF-81-NH2. The pseudo-first-order adsorption model of Lagergren inspected further kinetic data. In Supplementary Figure 7, the representation of linear fitting curves is given. Adsorption of 2-NO, PCMX, and BPA onto PAF-82-NH2, PAF-81-NH2, and PAF-80-NH2 to time could be better explained by the pseudo-first-order model, according to the values of the correlation coefficients (R2) and rate constant (Kobs). Supplementary Table 2 summarizes calculated parameters for kinetics and adsorption rates. Another adsorption model was applied to calculate maximum adsorption capacity and understand adsorbate and adsorbent interaction. The adsorption isotherms are drawn as a result of 2-NO, PCMX, and BPA adsorption onto PAF-82-NH2, PAF-81-NH2, PAF-80-NH2, and PAF-1 as shown in Supplementary Figures 8-10. Langmuir isotherm model was used exclusively for the analysis of obtained isotherm data. The Langmuir linear plots are represented in Supplementary Figures 11-13. The summary of calculated isotherm parameters is shown in Table 2. By considering the R2 value, the adsorption isotherms of the model OMPs were better studied by the Langmuir model. Langmuir isotherm model explains the adsorption pattern as monolayer adsorption and homogeneous sites within the absorbent[39]. It is observed that amine-functionalized PAF materials behaved differently in terms of adsorption towards three model micropollutants. The highest adsorption capacity of 763 mg·g-1 exhibited by PAF-82-NH2 for BPA is shown in Table 2. On the other hand, the adsorption capacities of PAF-80-NH2 and PAF-81-NH2 for the same pollutant BPA are 574 and 529 mg·g-1, respectively. In contrast, PAF-1 showed the lowest uptake capacity for BPA among all three amine-functionalized PAFs, which is 324 mg·g-1. About the uptake capacity of the second pollutant 2-NO, once again, the maximum adsorption capacity was showed by PAF-82-NH2 of
Langmuir isotherm model parameters for the adsorption of OMPs by PAFs
| Sorbent | Pollutant | qmaxa (mg·g-1) | Kb (L·mol-1) | R2c |
| PAF-80-NH2 | BPA | 574 | 22.2 | 0.998 |
| PAF-81-NH2 | 529 | 23.1 | 0.991 | |
| PAF-82-NH2 | 763 | 15.1 | 0.997 | |
| PAF-1 | 324 | 38.6 | 0.990 | |
| PAF-80-NH2 | 2-NO | 286 | 67.1 | 0.997 |
| PAF-81-NH2 | 250 | 72.5 | 0.991 | |
| PAF-82-NH2 | 461 | 28.1 | 0.997 | |
| PAF-1 | 245 | 17.1 | 0.998 | |
| PAF-80-NH2 | PCMX | 345 | 793.7 | 0.991 |
| PAF-81-NH2 | 301 | 49.0 | 0.986 | |
| PAF-82-NH2 | 497 | 278.6 | 0.996 | |
| PAF-1 | 44.2 | 23.5 | 0.991 |
The difference in the adsorption capacity of amine-functionalized PAF materials is linked with different specific surface areas of these materials. PAF-82-NH2 exhibits the fastest removal efficiency and highest adsorption capacity with the highest BET against three model contaminants once the polarity is developed in the form of amine functional groups on PAF channel walls. That is why efficient adsorption performance of PAF-82-NH2 is associated with its higher BET than PAF-80-NH2 and PAF-81-NH2.
In this case, the high BET of PAF-82-NH2 enhanced the interaction probability between adsorbate and adsorbent. On the other hand, a strong affinity with polar OMPs is developed with the help of the polar amine group at the framework’s channel for efficient adsorption performance.
Upon exploration of the reasons behind efficient adsorption of PAF-82-NH2, we also find that this material has a higher degree of conjugation in its framework than the other two PAFs, supported by Supplementary Figure 6. All three model pollutants are phenolic, possessing benzene rings in their structures, so excessive benzene rings in the PAF-82-NH2 framework interact through π-π interactions with the benzene rings of model phenolic pollutants. Through these π-π interactions, PAF-82-NH2 exhibits the best adsorption performance.
Likewise, after PAF-82-NH2, PAF-80-NH2 showed better adsorption performance than PAF-81-NH2, as the specific surface area of PAF-80-NH2 is higher than that of PAF-81-NH2. This study found that efficient adsorption performance is linked to the BET of porous materials when pore polarity is already established, just as seen in amine-functionalized PAFs.
Focusing on the adsorption mechanism, it has been seen that establishing functionalization at the pores of the framework leads to good adsorption due to hydrogen bonding interactions. The role of the -NH2 group for the adsorption of pollutants in porous frameworks has been explored in some previous studies, such as nanoUiO-66-NH2, where it has been observed that the presence of the -NH2 group at the pore of MOFs enhances the adsorption by hydrogen bonding when comes in contact with polar pollutants. Likewise, in our study, NH2 groups in amine-functionalized PAFs contribute significantly to the observed adsorption capacity for model OMPs. This can be attributed to the strong hydrogen bonding interactions between the NH2 group and the polar functional groups (-OH) in the OMPs[40]. FTIR technique was used to analyze if there is any band shift before and after adsorption of model OMPs by PAF-82-NH2. Upon observation, there were band shifts related to the amine group (N-H stretching) in the absorption of individual pollutants, supporting host-guest interactions such as hydrogen bonding, as mentioned earlier. More specifically, there is shift from 3,446 to 3,121 cm-1 for BPA@PAF-82-NH2, 3,446 to 3,127 cm-1 for 2-NO@PAF-82-NH2, and 3,446 to 3,123 cm-1 for PCMX@PAF-82-NH2, respectively
In addition, the pore size of the adsorbent can also be an influencing factor regarding contaminant adsorption. Therefore, PAF-82-NH2 pore size was compared with the molecular dimensions of model OMPs to analyze pore accessibility. Supplementary Figure 15 and Supplementary Table 3 depict a comparative analysis of the molecular dimensions of PCMX, 2-NO, and BPA. This reveals that PCMX
We also studied the adsorption mechanism of PAF-82-NH2 with BPA to gain insight into their adsorptive interactions at the atomic level via density functional theory (DFT) first-principles calculations through the Vienna Ab initio Simulation Package (VASP). We optimized the bare BPA and bare PAF-82-NH2 and then combined the structure of BPA adsorbed onto PAF-82-NH2. The adsorption energy of the combined structure of PAF-82-NH2 and BPA is calculated as -0.21 eV, according to
Here, EPAF is the total energy of the bare PAF-82-NH2, and EBPA is associated with the energy of isolated pollutant molecules (BPA). EPAF+BPA is the total energy of the PAF-82-NH2 supercell containing BPA. Meanwhile, the negative value of adsorption energy supports the thermodynamic feasibility of the adsorption phenomenon. The amine group plays a vital role in adsorption, providing active sites for electrostatic interactions, such as hydrogen bonding, as shown in Supplementary Figure 16. Another evidence of the involvement of the amine group in providing adsorptive sites for incoming OMPs is supported by the (N-H) band shift [Supplementary Figure 14]. DFT calculations support the dominance of physisorption phenomena, confirming the ability of amine-functionalized PAF to adsorb pollutants, gases, or other molecular polar contaminants.
High BET and smaller size of pores associated with the PAF framework may lead to maximum adsorption capacity and faster removal rates, as seen in the case of PAF-80-NH2 compared to PAF-81-NH2. By focusing on adsorption experimental results and amine-functionalized PAF features, we deduce that the exceptional performance of PAF-82-NH2 in terms of maximum adsorption capacity and fastest removal rates towards model OMPs is not governed by a solo factor but the combination of various factors acting together, for instance, the specific surface area of adsorbent, pore size of framework, polarity matching between adsorbent and adsorbate due to amine functional groups, and degree of conjugation in framework.
In addition, PAF-82-NH2 surpasses the adsorption capacities of PAF-82[22], despite having lower BET than PAF-82 (1,073 m2·g-1), proving the potential of the amine group to enhance the adsorption capacity of PAF materials. Supplementary Table 4 highlighted the adsorption capacities of some recently reported adsorbents and their comparison with this study. Comparing these obtained adsorption capacities of amine-functionalized PAFs with hydroxyl-functionalized PAFs, we found that no matter having low surface area compared to hydroxyl PAFs, amine-functionalized PAFs showed higher adsorption capacities. This signifies that the amine group can enhance the adsorption capacity for micropollutants, developing a better polarity match between PAF and pollutants. PAF-82 also contains polar functional groups (-OH) by considering its structure given in Supplementary Figure 17. However, in our study, the amine group may induce better polarity matching between phenolic pollutants and pore channels, thus performing better than the hydroxyl group carrying PAF-82. Our study gives insights into developing amine-functionalized porous frameworks for the high adsorption capacity and fast removal of hazardous micropollutants for future work.
Similarly, PAF-1 bearing an exceptionally high BET of 5,600 m2·g-1 could not even surpass the adsorption capacity of (PAF-81-NH2), amine-functionalized PAF with the lowest BET of 564 m2·g-1 for all the three model micropollutants. We learned that surface area does not majorly influence the adsorption of micropollutants as a single factor. The polarity-matching strategy between PAFs and pollutants should be prioritized first by introducing suitable polar functional groups in the aromatic hydrophobic backbone of porous materials. Once polarity is developed among channel walls of porous materials, the surface area seems activated with other factors such as pore size and conjugation. Then, we can expect adsorption capacity according to surface area in a linear relationship.
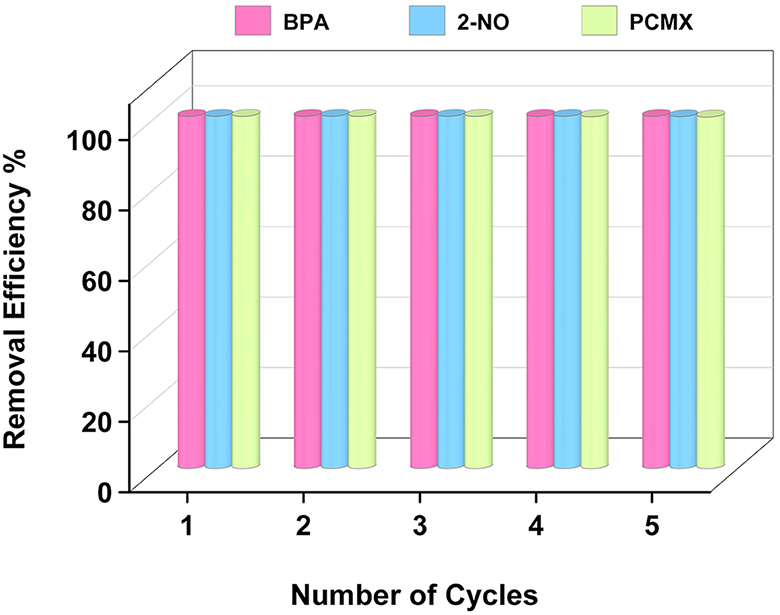
Another factor considered significant for evaluating adsorption performance is the recyclability of the adsorbent. For the sake of desorption of model pollutants that were adsorbed by amine-functionalized PAF materials, ethanol (EtOH) can be used to remove OMPs from the porous framework. To evaluate the recyclability of best-performing amine-functionalized PAF-82-NH2, adsorption/desorption experiments were performed five times consecutively. During each adsorption, the removal efficiency of PAF materials was calculated. Results of the recycling test showed that after five cycles of adsorption/desorption, the adsorption capacity of PAF-82-NH2 remained the same without any loss in its capacity, as represented in Figure 5. This test demonstrates the outstanding capacity for recycling PAF-82-NH2 towards the adsorption of model OMPs when they come in contact with water.
Figure 5. OMPs removal efficiency histogram of PAF-82-NH2. OMPs: Organic micropollutants; PAF: porous aromatic framework; BPA: bisphenol-A; PCMX: para-chloro-meta-xylenol.
PAF-82-NH2 is designed to be a cost-effective yet outstanding adsorbent for hazardous OMPs by exploiting affordable precursor 2,4,6-tribromoaniline compared to other functionalized porous materials. Our material is not as cheap as activated carbon[41], which has been employed in wastewater treatment plants.
Based on TGA, amine-functionalized PAF materials have good stability, so the heating method can also remove adsorbed pollutants. Different procedures can be utilized to recycle adsorbed amine-functionalized PAF materials depending on the ease of operation and practical requirements.
However, PAF-82-NH2 demonstrated significantly higher adsorption capacities for selected OMPs due to its tailored functional groups, which enable specific interactions such as hydrogen bonding. Furthermore, PAF-82-NH2 can be regenerated and reused for multiple cycles without significant loss of efficiency, reducing the overall operational cost. These features make it a promising material for specialized applications, such as treating industrial wastewater or targeted pollutant removal as environmental remediation. On an industrial scale, contaminated water treatment plants can be installed with amine-functionalized PAFs as adsorbent membranes for the adsorption of diverse hazardous OMPs. However, some real-life challenges must be dealt with, such as the long-term stability of amine-functionalized PAFs under real-world conditions, as these PAFs have already shown promising stability under laboratory conditions. Cost-effective, scalable methods must be explored to make amine-functionalized PAFs commercially viable for widespread use in environmental applications.
CONCLUSIONS
We reported three novel amine-functionalized PAFs (PAF-82-NH2, PAF-81-NH2, PAF-80-NH2) featuring cost-effective precursor 2,4,6-tribromoaniline. Characterization techniques (FTIR, SEM, BET) confirmed successful synthesis. In adsorption analysis, PAF-82-NH2 exhibited maximum capacity and fastest removal efficiency compared to the others, showcasing the highest BET (806 m2·g-1), optimal pore size, and high conjugation. PAF-82-NH2 uptake for BPA, 2-NO, and PCMX was 763, 461, and 497 mg·g-1, respectively, surpassing most prior absorbents. The -NH2 group significantly enhances adsorption capacity via hydrogen bonding, outperforming the -OH group. We have observed that specific surface area alone is insufficient; pore polarity leads to efficient adsorption, coordinating BET, conjugation, and pore size.
DECLARATIONS
Authors’ contributions
Designed the experiments, synthesized materials, carried out characterization, interpreted data, concluded results, drew the figures, and wrote the manuscript: Javaid, A.
Conducted characterization techniques and data analysis: Ahmad, T.; Lei, H.
Conducted DFT calculations and helped in data analysis: Zhu, C.
Helped in kinetic experiments: Lei, H.; Shah, N.A.
Conducted contact angle measurements and helped in analytical discussion: Dou, Z.
Conducted UV-vis absorbance readings and PXRD: Ding, Y.; Li, Y.
Directed and supervised the project, analyzed data, revised the manuscript, and provided administrative and technical support: Tian, Y.
Availability of data and materials
Data supporting our findings, evidence of characterization techniques, and models for adsorption experiments can be found in this article and its Supplementary Materials. The corresponding author can be contacted for any additional data needs if required.
Financial support and sponsorship
The authors are grateful for financial support from the National Key R&D Program of China (Grant No. 2022YFB3805900, No. 2022YFB3805902), the National Natural Science Foundation of China (Grant No. 22075040), and the “111” project (No. B18012).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Guo, Z.; Kodikara, D.; Albi, L. S.; et al. Photodegradation of organic micropollutants in aquatic environment: importance, factors and processes. Water. Res. 2023, 231, 118236.
2. Sanchez-huerta, C.; Baasher, F.; Wang, C.; Zhang, S.; Hong, P. Global occurrence of organic micropollutants in surface and ground water: highlighting the importance of wastewater sanitation to tackle organic micropollutants. J. Environ. Chem. Eng. 2024, 12, 112861.
3. der Hoek JP, Deng T, Spit T, Luimstra V, de Kreuk M, van Halem D. Bromate removal in an ozone-granular activated carbon filtration process for organic micropollutants removal from wastewater. J. Water. Process. Eng. 2024, 58, 104877.
4. Tarafdar, A.; Sirohi, R.; Balakumaran, P. A.; et al. The hazardous threat of bisphenol A: toxicity, detection and remediation. J. Hazard. Mater. 2022, 423, 127097.
5. Lin, T. J.; Guo, Y. L.; Hsu, J. C.; Wang, I. J. 2-naphthol levels and allergic disorders in children. Int. J. Environ. Res. Public. Health. 2018, 15, 1449.
6. Au, C. K.; Jason, C. K. K.; Chan, W.; Zhang, X. Occurrence and stability of PCMX in water environments and its removal by municipal wastewater treatment processes. J. Hazard. Mater. 2023, 445, 130550.
7. Alayan, H. M.; Aljumaily, M. M.; Alsaadi, M. A.; Mjalli, F. S.; Hashim, M. A. A review exploring the adsorptive removal of organic micropollutants on tailored hierarchical carbon nanotubes. Toxicol. Environ. Chem. 2021, 103, 282-325.
8. Haidar, O.; Roques-carmes, T.; Gouda, A.; Tabaja, N.; Toufaily, J.; Hmadeh, M. Defect-rich metal-organic framework nanocrystals for removal of micropollutants from water. ACS. Appl. Nano. Mater. 2024, 7, 10003-15.
9. Bakare, B. F.; Adeyinka, G. C. Occurrence and fate of triclosan and triclocarban in selected wastewater systems across Durban Metropolis, KwaZulu-Natal, South Africa. Int. J. Environ. Res. Public. Health. 2022, 19, 6769.
10. Reddy, N. D.; Elias, A. J. Chlorine and the chemistry of disinfectants: a fascinating journey - 18th century to the COVID times. Reson 2021, 26, 341-66.
11. Zawadzki, P.; Kudlek, E.; Dudziak, M. Kinetics of the photocatalytic decomposition of bisphenol A on modified photocatalysts. J. Ecol. Eng. 2018, 19, 260-8.
12. Cruz, J. V.; Magalhães, W. L. E.; Cademartori, P. H. G.; Dorta, D. J.; De, O. D. P.; Leme, D. M. Environmental concerns about the massive use of disinfectants during COVID-19 pandemic: an overview on aquatic toxicity. Ecotoxicol. Environ. Contam. 2021, 16, 107-17.
13. Abdullah; U. A.A.U.; Hanapi, N.S.M.; Wan Ibrahim, W.N.; et al. Micro-solid phase extraction (μ-SPE) based on alginate/multi-walled carbon nanotubes sorbent for the determination of bisphenol A in canned fruits. Chiang Mai J Sci 2018, 45, 2348-60. https://www.scopus.com/record/display.uri?eid=2-s2.0-85056382105&origin=inward&txGid=3a2e7b6f4258ac5264a06ea2e155030d (accessed 2025-05-26).
14. Brahma, B.; Sen, S.; Sarkar, P.; Sarkar, U. Interference-free electrocatalysis of p-chloro meta xylenol (PCMX) on uniquely designed optimized polymeric nanohybrid of P(EDOT-co-OPD) and fMWCNT modified glassy carbon electrode. Anal. Chim. Acta. 2021, 1168, 338595.
15. Mishra, A.; Goel, D.; Shankar, S. Bisphenol A contamination in aquatic environments: a review of sources, environmental concerns, and microbial remediation. Environ. Monit. Assess. 2023, 195, 1352.
16. Krugly, E.; Martuzevicius, D.; Tichonovas, M.; et al. Decomposition of 2-naphthol in water using a non-thermal plasma reactor. Chem. Eng. J. 2015, 260, 188-98.
17. Han, J.; Li, W.; Zhang, X. An effective and rapidly degradable disinfectant from disinfection byproducts. Nat. Commun. 2024, 15, 4888.
18. Yang, Y.; Zhang, X.; Jiang, J.; et al. Which micropollutants in water environments deserve more attention globally? Environ. Sci. Technol. 2022, 56, 13-29.
19. Peng, J.; Yin, R.; Yang, X.; Shang, C. A novel UVA/ClO2 advanced oxidation process for the degradation of micropollutants in water. Environ. Sci. Technol. 2022, 56, 1257-66.
20. Wang, J.; Poursat, B. A. J.; Feng, J.; et al. Exploring organic micropollutant biodegradation under dynamic substrate loading in rapid sand filters. Water. Res. 2022, 221, 118832.
21. Antonopoulou, M.; Bika, P.; Papailias, I.; et al. Photocatalytic degradation of organic micropollutants under UV-A and visible light irradiation by exfoliated g-C3N4 catalysts. Sci. Total. Environ. 2023, 892, 164218.
22. Mo, C.; Faheem, M.; Aziz, S.; et al. Hydroxyl porous aromatic frameworks for efficient adsorption of organic micropollutants in water. RSC. Adv. 2020, 10, 26335-41.
23. Wang, P.; An, G.; Jarvis, P.; et al. Simultaneous removal of organic micropollutants and metals from water by a multifunctional β-cyclodextrin polymer-supported-polyaniline composite. Chem. Eng. J. 2024, 482, 148826.
24. Drenkova-Tuhtan, A.; Inskeep, C. S.; Luthardt, L.; et al. Reusable and inductively regenerable magnetic activated carbon for removal of organic micropollutants from secondary wastewater effluents. Water. Res. 2024, 255, 121525.
25. Zheng, X.; Jiang, N.; Zheng, H.; Wu, Y.; Heijman, S. G. Predicting adsorption isotherms of organic micropollutants by high-silica zeolite mixtures. Sep. Purif. Technol. 2022, 282, 120009.
26. Zhao, Y.; Zhao, Y.; Wu, C.; et al. An ultrastable crystalline acylhydrazone-linked covalent organic framework for efficient removal of organic micropollutants from water. Chemistry 2021, 27, 9391-7.
27. Guo, W.; Liu, J.; Tao, H.; et al. Covalent organic framework nanoarchitectonics: recent advances for precious metal recovery. Adv. Mater. 2024, 36, e2405399.
28. Liu, K.; Liu, Y.; Wu, Y.; et al. Advances in reticular materials for sustainable rare earth element recovery. Coord. Chem. Rev. 2025, 522, 216199.
29. Garg, R.; Sabouni, R.; Alaamer, A.; et al. Recent development in metal-organic framework-based hybrid nanocomposites for pollutants remediation from wastewater: challenges and opportunities. Environ. Technol. Innov. 2023, 32, 103446.
30. Ge, S.; Wei, K.; Peng, W.; et al. A comprehensive review of covalent organic frameworks (COFs) and their derivatives in environmental pollution control. Chem. Soc. Rev. 2024, 53, 11259-302.
31. Ben, T.; Qiu, S. Porous aromatic frameworks: synthesis, structure and functions. CrystEngComm 2013, 15, 17-26.
32. Shen, X.; Faheem, M.; Matsuo, Y.; et al. Polarity engineering of porous aromatic frameworks for specific water contaminant capture. J. Mater. Chem. A. 2019, 7, 2507-12.
33. Castaldo, R.; Avolio, R.; Cocca, M.; Errico, M. E.; Avella, M.; Gentile, G. Amino-functionalized hyper-crosslinked resins for enhanced adsorption of carbon dioxide and polar dyes. Chem. Eng. J. 2021, 418, 129463.
34. Ben, T.; Ren, H.; Ma, S.; et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. Engl. 2009, 48, 9457-60.
35. Rudzinski, W.; Plazinski, W. Kinetics of solute adsorption at solid/aqueous interfaces: searching for the theoretical background of the modified pseudo-first-order kinetic equation. Langmuir 2008, 24, 5393-9.
36. García-Zubiri, I. X.; González-Gaitano, G.; Isasi, J. R. Sorption models in cyclodextrin polymers: Langmuir, Freundlich, and a dual-mode approach. J. Colloid. Interface. Sci. 2009, 337, 11-8.
37. Byun, Y.; Coskun, A. Bottom-up approach for the synthesis of a three-dimensional nanoporous graphene nanoribbon framework and its gas sorption properties. Chem. Mater. 2015, 27, 2576-83.
38. Seo, P. W.; Khan, N. A.; Hasan, Z.; Jhung, S. H. Adsorptive removal of artificial sweeteners from water using metal-organic frameworks functionalized with urea or melamine. ACS. Appl. Mater. Interfaces. 2016, 8, 29799-807.
39. Butyrskaya, E. Understanding the mechanism of monolayer adsorption from isotherm. Adsorption 2024, 30, 1395-406.
40. Rojas, S.; Torres, A.; Dato, V.; et al. Towards improving the capacity of UiO-66 for antibiotic elimination from contaminated water. Faraday. Discuss. 2021, 231, 356-70.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data



















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.