Nitrogen and oxygen-codoped dense porous carbon enhancing ion adsorption for high-volumetric performance supercapacitors
Abstract
The increasing prevalence of portable electronics and Internet of Things devices has led to a rising demand for energy storage devices that can charge and discharge quickly. Supercapacitors, known for their high power density and long working life, are considered as promising candidates. Nevertheless, the comparatively low energy density continues to pose a substantial challenge. Activated carbon, despite its widespread use as the electrode material, still suffers from unsatisfactory specific volumetric capacitance. Here, we propose a novel co-chemical welding strategy using coal liquefaction residue-derived carbon dots and melamine as precursors, developing a nitrogen/oxygen-codoped dense porous carbon with carbon dot-embedded amorphous carbon structure via electrostatic assembly and mechanical compaction. The optimized material achieves a high compaction density of 1.19 g cm-3, providing continuous conductive pathways and additional active sites due to nitrogen/oxygen (N/O) co-doping. Our experimental results demonstrate remarkable improvements in both volumetric and gravimetric specific capacitances, reaching 373.6 F cm-3 and 314 F g-1 at a current density of 1 A g-1, respectively. Density functional theory results also confirm that N/O co-doping enhances ion adsorption capacity. This study may provide a new approach for developing high-volumetric capacitance supercapacitor electrode materials, thereby advancing the field of energy storage technologies.
Keywords
INTRODUCTION
Demand for energy storage devices with quick charging/discharging is rising as portable electronics and Internet of Things (IoT) devices become more common[1-3]. Supercapacitors have garnered significant attention due to their high power density, long lifespan, high safety, and simple structure[4,5]. However, their relatively low volumetric energy density limits their practical applications. Activated carbon (AC), characterized by its large specific surface area (SSA), good electrical conductivity, and excellent electrochemical stability, has become a widely used electrode material for supercapacitors[6]. Nevertheless, the specific capacitance of AC remains suboptimal for practical applications. Enhancing the specific capacitance of AC is often achieved by increasing the SSA, and numerous activation techniques have been documented to attain high SSA levels[7-9]. Although a higher SSA positively influences capacitance, it is not the sole factor influencing performance. The distribution of pore sizes also significantly affects the functionality of carbon materials[10]. Mesopores generally facilitate ion diffusion by providing effective pathways, whereas micropores play a crucial role in boosting capacitance[11-13]. Excessive porosity in high-SSA materials often leads to volumetric expansion and reduced material density (< 0.6 g·cm-3), lowering effective ion-accessible content per unit volume[14,15]. This makes it challenging for volumetric specific capacity to meet the future development requirements of high energy density, miniaturization, and the lightweight design for supercapacitors. Therefore, achieving a hierarchical porous architecture that incorporates both mesopores and micropores while enhancing volumetric specific capacitance can effectively improve surface area utilization.
To address the issues of low density and non-connected channels in AC, researchers have proposed strategies such as homogenous activation under mechanical compaction, graphene-induced densification assembly, and ultra-microchannel design to increase density[16-18]. Among these, homogenous activation under mechanical compaction eliminates inter-particle voids and promotes the activation process, resulting in higher porosity, interconnected porous structure and compaction density compared to traditional AC. This provides an effective and straightforward method for preparing high-density AC. However, this approach reduces SSA and causes partial loss of micropores during activation, leading to a decrease in specific capacitance. Thus, further modification of AC is necessary to achieve both high density and high specific capacitance.
The increase in compaction density leads to several effects. (1) Enhancement of volumetric specific capacitance. As the compacted density increases, the mass of AC per unit volume rises, resulting in an improvement in volumetric energy density; (2) Reduction of internal resistance and improvement of conductivity. The interparticle contact becomes tighter, and the conductive network becomes more complete, thereby reducing internal resistance and enhancing power performance. However, excessively high compacted density may compress pore channels, reduce porosity, hinder electrolyte impregnation, and restrict ion diffusion, which can ultimately lead to a decline in power performance[17,18]. Therefore, a balance exists between compacted density and pore structure. Rationally controlling both parameters and identifying the optimal balance point among conductivity, capacity, power performance, and cycle life is crucial for the design of supercapacitor electrodes.
The capacitance performance of AC is typically optimized via heteroatom doping, a method widely recognized for its high efficiency[19,20]. More precisely, nitrogen doping, which takes advantage of the comparable atomic size of nitrogen and carbon and the higher electronegativity of nitrogen (3.04 vs. 2.55 for carbon), enhances polarization in C-N bonds. This, in turn, promotes the adsorption of electrolyte ions[21]. Furthermore, boosting the concentration of oxygen-based surface functional groups can significantly improve pseudocapacitance contributions within a specific range[22,23]. Heteroatom doping plays a crucial role in enhancing pseudocapacitive behavior. The introduction of heteroatom-containing functional groups serves dual purposes: reducing interfacial charge transfer resistance in carbon-based electrodes while simultaneously improving electrolyte infiltration within the porous carbon matrix[24-26]. This synergistic effect significantly optimizes charge carrier mobility across the graphitic lattice structure. Despite its effectiveness in enhancing performance, conventional heteroatom doping methods relying on biomass or synthetic resins suffer from high costs and limited scalability. Identifying new, low-cost carbon sources thus represents a critical direction[27-29]. Building upon prior research, coal liquefaction residue (CLR) was employed as a precursor material in our studies[30]. We synthesized carbon dots (CDs) with oxygen-rich surface groups and high production efficiency using optimized chemical fragmentation techniques. When used as electrode materials, these nanomaterials showed excellent electrochemical performance and superior charge storage capacity. This method not only repurposes industrial byproducts but also offers an environmentally sustainable way to synthesize advanced materials.
Herein, we use CLR-derived CDs as structural units and the amino group in melamine molecules as the nitrogen doping source. Through electrostatic assembly, CDs and melamine are uniformly dispersed. Mechanical compaction constructs CDs-embedded amorphous carbon (CDEA) structure, forming a three-dimensional conductive network. Thanks to the unique CDEA structure, this material forms excellent conductive pathways in the amorphous carbon matrix, significantly reducing electrode impedance. Moreover, the uniform co-activation and nitrogen/oxygen (N/O) co-doping strategy not only increases the material’s compaction density to 1.19 g cm-3 but also endows it with a through-hole structure and additional N/O co-doped active sites. When evaluated in a three-electrode configuration, the material demonstrates remarkable volumetric (373.6 F cm-3) and gravimetric (314 F g-1) capacitance values at 1 A g-1 current density, markedly exceeding the performance metrics of unmodified dense porous carbon (DPC) counterparts (262 F cm-3 and 254.1 F g-1). In a two-electrode configuration, it demonstrates a specific capacitance of 350.6 F cm-3 (294.6 F g-1) at a current density of 1 A g-1. The assembled nitrogen/oxygen-codoped dense porous carbons (NDPC)//NDPC supercapacitor delivers an energy density of 12.1 Wh L-1 (10.2 Wh kg-1) with corresponding power density of 148.0 W L-1 (124.4 W kg-1) in 6 M KOH electrolyte, while demonstrating enhanced performance in organic electrolyte with 35.3 Wh L-1 (29.7 Wh kg-1) energy density at 711.1 W L-1 (597.3 W kg-1) power density. Density functional theory (DFT) calculations reveal that the incorporation of N/O co-doping within the carbon matrix significantly enhances the K+ adsorption energy through optimized electronic structure and improved charge distribution characteristics.
EXPERIMENTAL SECTION
All chemical reagents were utilized as received, without undergoing additional purification or treatment. The CDs were synthesized via a chemical oxidation approach in an H2O2 and H2SO4 system, using CLR provided by the Shenhua Ordos Coal and Oil Branch. Detailed procedures can be found in our previous works[30].
Synthesis of nitrogen/oxygen-codoped dense porous carbons (NDPCs)
In a standard synthesis process, 0.5 g of CDs and 0.25 g of melamine were dispersed in 60 mL of ethanol to form solution A through ultrasonic treatment for 15 min. A specific quantity of KOH (equal to half the combined mass of CDs and melamine) was dissolved in 20 mL of ethanol to prepare solution B. Solutions A and B were then thoroughly mixed using magnetic stirring for 12 h. The resulting mixture was dried at
Characterization
N2 adsorption-desorption isotherms were analyzed using an ASAP 2460 instrument (Micromeritics). SSA and pore size distribution were evaluated using the Braunauer-Emmett-Teller (BET) method and Non-Local Density Functional Theory (NLDFT). X-ray diffraction (XRD) measurements were performed on a Bruker D8 Advance system. Raman spectroscopy was conducted with a Bruker SENTERRA dispersive Raman spectrometer, utilizing a laser wavelength of 532 nm. Surface elemental composition was examined via X-ray photoelectron spectroscopy (XPS, Thermo Scientific ESCALAB Xi+), with experimental data provided by eceshi (www.eceshi.com). Sample morphology and structural characteristics were analyzed using scanning electron microscopy (SEM, Hitachi S-4800) and high-resolution transmission electron microscopy (HRTEM, FEI Talos F200x). Electrochemical test, compaction density measurement, and DFT calculation details can be found in Supplementary Notes 1-3.
RESULTS AND DISCUSSION
Preparation and morphology structure characterization of NDPCs
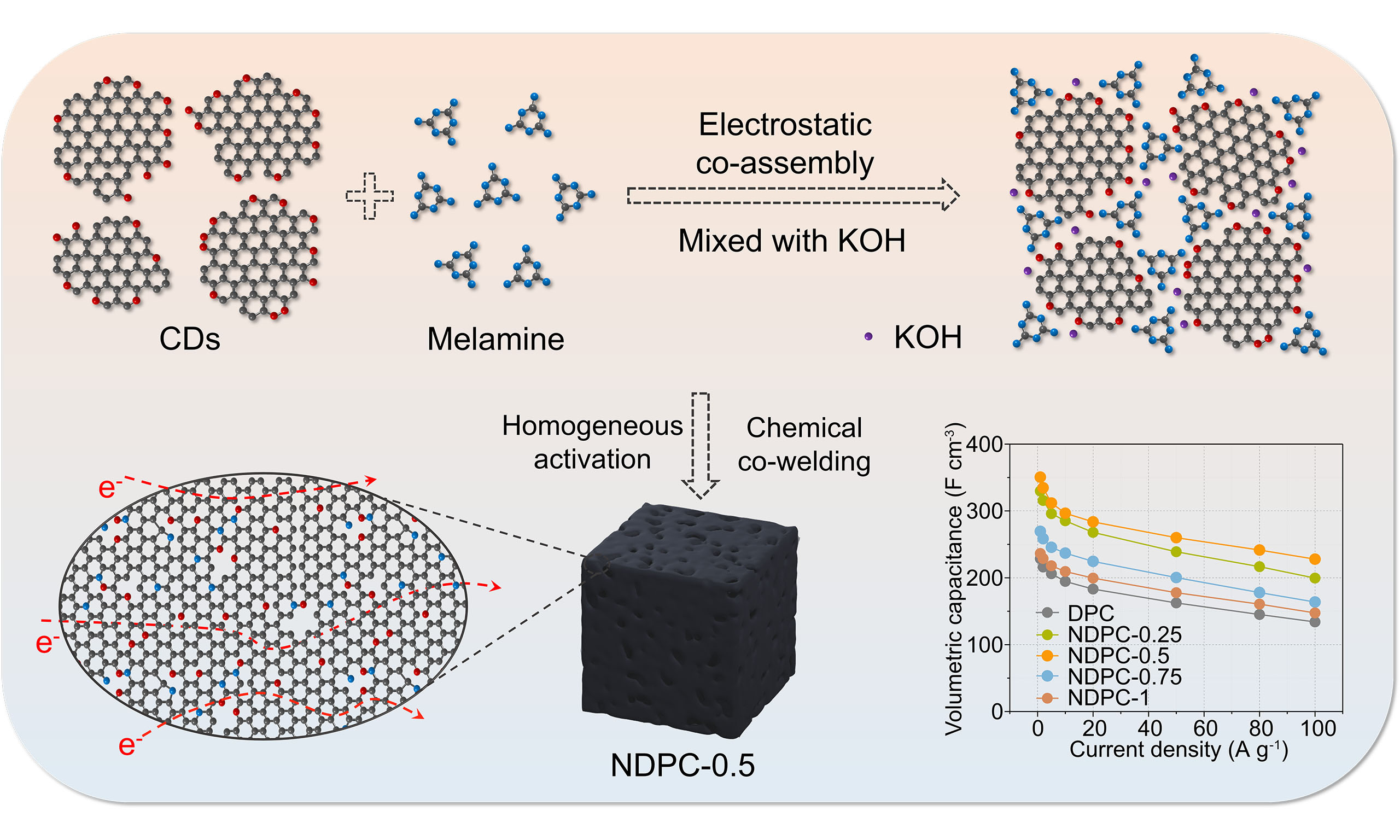
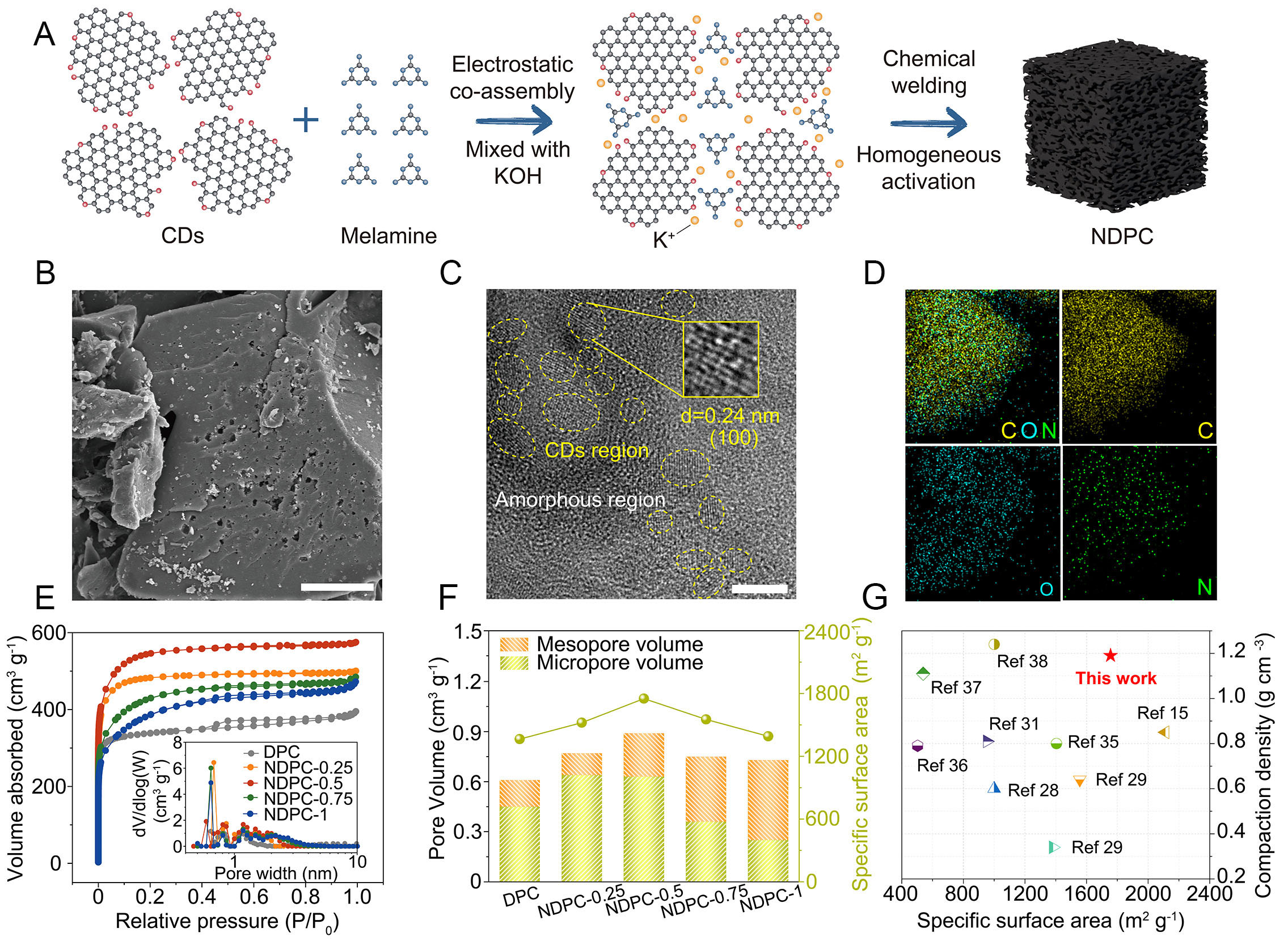
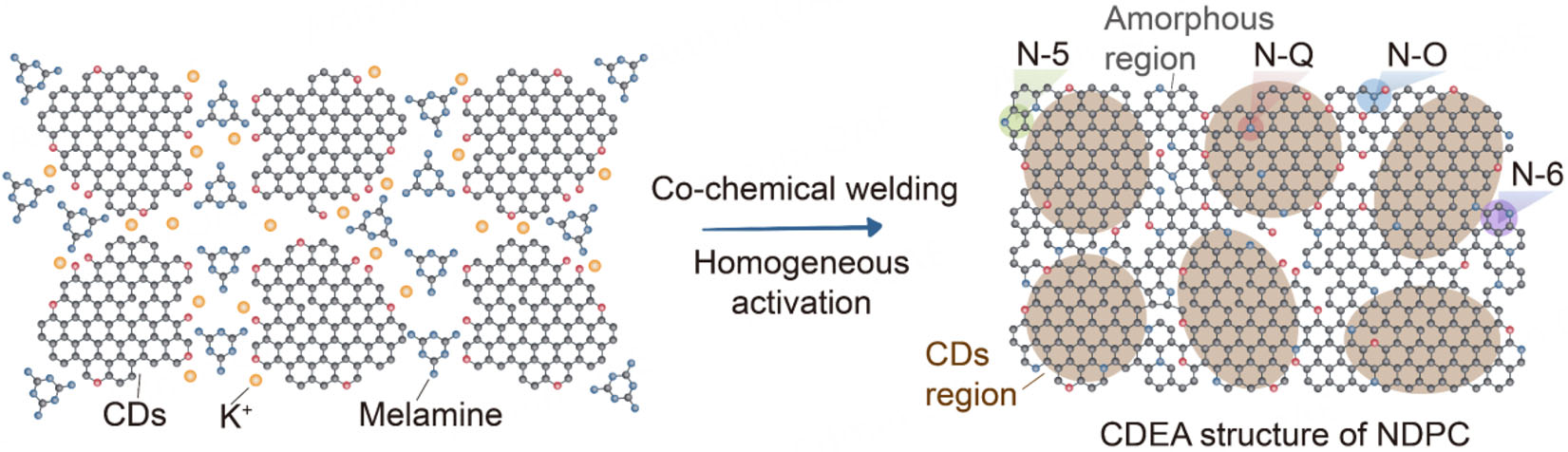
Figure 1A demonstrates the effective fabrication of N/O co-doped CDEA-architected NDPC via a single-step carbonization process coupled with uniform activation. This process involved the electrostatic co-assembly of CDs and melamine, followed by mechanical compaction. The morphology of the NDPCs was obtained by SEM. As can be seen, all samples display a similar and dense bulk structure, with particle sizes much larger than those of the precursors (CDs), indicating that NDPCs were formed by the agglomeration and co-welding of CDs and melamine during the high-temperature treatment [Supplementary Figure 1]. The reaction can be given as follows[30]:
Figure 1. Preparation process, morphology and pore distribution of NDPCs. (A) Illustration of the preparation process and structure of NDPC-0.5. (B) SEM images (scale bar: 10 μm), (C) HRTEM (scale bar: 5 nm) and (D) EDS elemental mapping images of NDPC-0.5. (E) N2 absorption-desorption isotherms and the corresponding pore size distribution plot (inset). (F) Pore volume and SSA of the NDPCs. (G) Comparison of SSA and compaction density of the NDPC-0.5 with other porous carbons.
From the enlarged image, it can be seen that the surface of the DPC is distributed with homogeneous micropores, and the presence of large pores is negligible [Supplementary Figure 1A-C]. As the amount of melamine increases, a greater number of pores become evident on the surface of the samples. NDPC-0.5 exhibits uniformly distributed micropores and mesopores on its surface [Figure 1B and
The microstructure of NDPCs was observed by HRTEM. As illustrated in Supplementary Figure 2, DPC demonstrates a highly amorphous structure, which results from the random aggregation of CDs during the homogeneous activation process. The NDPCs exhibit highly crystalline regions internally, which are uniformly dispersed throughout the carbon layer [Supplementary Figure 2]. The d-spacing of the microcrystals is approximately 0.24 nm, exceeding the lattice spacing of pristine CDs (0.21 nm) [Figure 1C, Supplementary Figure 3]. This phenomenon can be explained by the introduction of nitrogen atoms, which help retain certain crystalline regions in CDs and result in an expanded interlayer spacing of microcrystals within the DPC[31-34]. Energy dispersive X-ray spectrometry (EDS) elemental mapping analysis [Figure 1D] reveals homogeneous spatial distribution of carbon, nitrogen, and oxygen constituents throughout the NDPC-0.5 architecture, thus confirming successful nitrogen doping within the material’s internal structure.
To investigate how nitrogen doping affects the material’s porous architecture, we conducted systematic assessments of surface characteristics and pore network configurations using nitrogen sorption analysis. The NDPCs exhibited characteristic type I adsorption curves [Figure 1E]. Within the low relative pressure regime (P/P0 < 0.1), the rapid rise in adsorption capacity suggests the presence of a rich abundance of micropores generated during the activation process. Within the intermediate-pressure range
To explain the high compaction density retained by NPDCs after mechanical compaction, we propose a pore structure development mechanism for ACs prepared through conventional outward-to-inward activation compared with mechanical compaction to enable homogeneous activation
Moreover, even after undergoing acid washing, grinding, and drying treatments, the compacted density of NDPC remains at a high level. This property is influenced by multiple factors, including particle size and internal pore structure. With respect to particle size, the particle size of NDPC (greater than 5 μm, Figure 1B, Supplementary Figure 1G-I) is significantly larger than that of its precursor CDs (approximately 5 nm, Supplementary Figure 3), a phenomenon closely associated with the co-activation and co-chemical welding processes. CDs, which are rich in oxygen-containing functional groups, can form assembly structures with melamine and KOH through electrostatic interactions and hydrogen bonding. Meanwhile, mechanical compaction generates compacted sheets that facilitate close contact between particles. During the co-chemical welding and co-activation process, CDs - serving as highly conjugated structural units - connect with melamine via their abundant oxygen-containing functional groups and edge active sites, ultimately forming large-sized particles with a CDEA structure. The significantly larger particle size of NDPC enhances its density substantially. Even after undergoing acid washing, grinding, and drying treatments, the particle size remains relatively large. Regarding pore structure, compared with traditional AC prepared via outward-to-inward activation, NDPC is predominantly composed of micropores and exhibits a relatively smaller SSA, which contributes to its higher compact density. The acid washing and drying procedures effectively remove impurities such as residual K metal particles from the pores without significantly compromising the original pore structure. Consequently, NDPC maintains a high compact density even after undergoing cleaning and drying treatments.
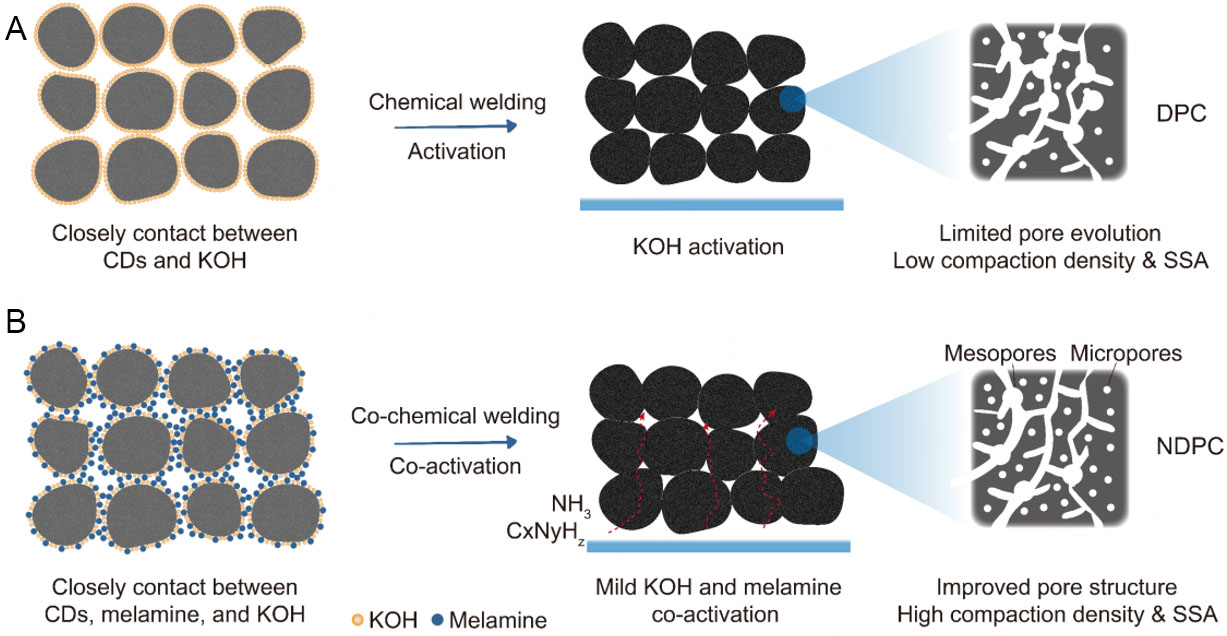
Compared with DPC, NDPC-0.5 exhibits a higher compaction density and a more developed microporous structure, which can be attributed to the following two factors [Scheme 1]: (1) During pyrolysis, melamine generates g-C3N4, which reacts with KOH and thereby mitigates the etching effect of KOH on the carbon precursor. Meanwhile, the application of mechanical pressure facilitates the formation of a dense structure, effectively suppressing the rapid release of active gas molecules (such as NH3, CxNyHz, etc.) during pyrolysis. This enables these species to remain within the carbon framework and continue participating in activation and nitrogen doping processes, thereby promoting the formation of abundant micropores and enhancing nitrogen doping efficiency. In contrast, during the synthesis of DPC, the absence of melamine protection results in stronger KOH etching, leading to micropore coalescence and collapse, which increases the formation of mesopores and consequently reduces the bulk density. (2) In the preparation of NDPC-0.5, condensation reactions between functional groups such as -COOH, -OH, and -NH2 induce chemical welding among carbon precursors, resulting in a more compact carbon matrix and achieving effective N/O co-doping. Such a co-chemical welding process is absent in the synthesis of DPC.
Scheme 1. Schematic of the porous structure development of the DPC and NDPC-0.5 through co-activation. (A) Porous structure development diagram of DPC, where CDs and KOH are in close contact (left), after KOH activation and chemical welding, forms a DPC (right) with a limited pore structure, a lower compaction density and SSA. (B) Porous structure development diagram of NDPC-0.5, where CDs, melamine and KOH are in close contact (left), after co-activation and co-chemical welding, forms an NDPC-0.5 (right) with an improveded pore structure, a higher compaction density and SSA.
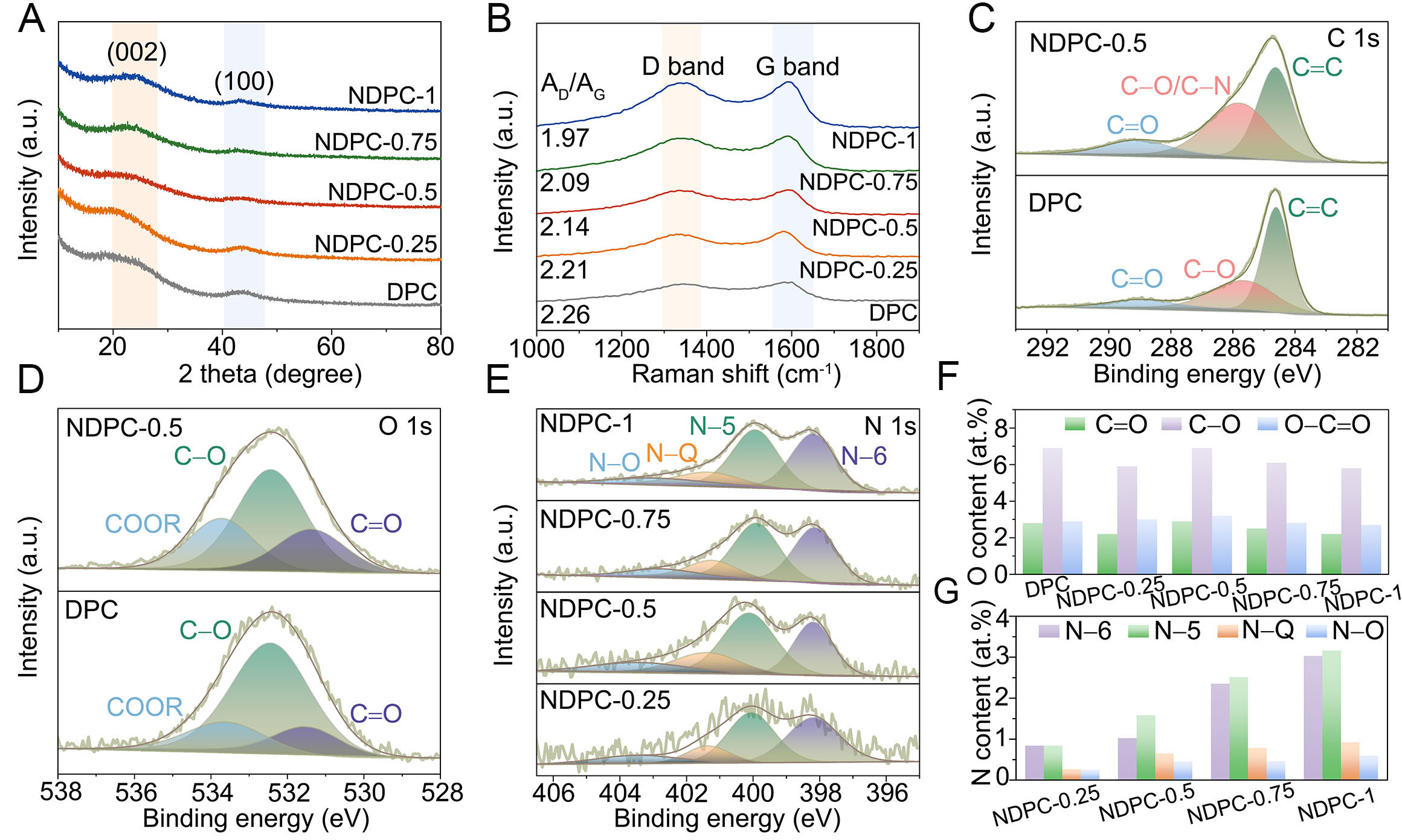
The XRD patterns provide insight into the crystallinity of the materials. In Figure 2A, all prepared samples display broad yet intense diffraction peaks near 23° and 44°, correlating with the (002) and (100) crystal planes of carbon-based materials[39-41]. Notably, as the melamine concentration rises, a progressive enhancement in peak intensity is observed for the (002) plane within the NDPC samples. This suggests that nitrogen doping serves as an important factor in substantially improving the crystallinity of AC. Raman spectrum further validates this observation [Figure 2B]. The AD/AG ratio, obtained by fitting the Raman peak areas, quantifies the material’s defect density [Supplementary Figure 5]. It is evident that with increasing melamine content, the AD/AG values decrease from 2.28 for DPC to 1.97 for NDPC-1, suggesting a reduction in defect density and an increase in ordered structures, which facilitates rapid electron transport. These findings are consistent with the HRTEM observations.
Figure 2. Phase characterization of the materials. (A) XRD patterns, and (B) Raman spectra of NDPCs. (C) XPS C 1s spectra and (D)
To explore the influence of nitrogen doping on the surface composition of NDPC, XPS was utilized. XPS survey spectra [Supplementary Figure 6] demonstrate consistent elemental profiles across all NDPC samples, with distinct C 1s, N 1s, and O 1s peaks observed in all samples except DPC. This observation aligns with EDS findings, verifying the effective nitrogen integration into the carbon matrix. The high-resolution C1s spectrum [Figure 2C] shows the presence of C-N bonds in NDPC-0.5, but not in the undoped DPC, confirming the incorporation of nitrogen. By deconvoluting the C 1s, O 1s [Figure 2D] and N 1s [Figure 2E] spectra, specific information regarding the types and quantities of C, O and N species can be obtained (available in Supplementary Tables 3 and 4). Figure 2F shows the contribution of O components in NDPCs; interestingly, the O content of NDPC-0.25 was 11.1 at.%, slightly lower than that of DPC (11.5 at.%). This suggests that at high temperatures, a limited amount of melamine fails to fully react with the oxygen functional groups in CDs, leading to the etching of these groups by KOH. For NDPC-0.75 and NDPC-1, the increased amount of KOH relative to CDs enhances the etching effect, thereby reducing the oxygen content. Notably, NDPC-0.5 exhibited the highest O content (13.0 at.%), indicating that an optimal amount of melamine addition during the co-chemical welding process effectively protects the oxygen functional groups in CDs. The nitrogen configurations N-5 and N-6 function as active sites for Faradaic processes, substantially improving capacitive characteristics, while N-Q and N-O configurations optimize charge transfer efficiency through enhanced electron mobility[42]. As shown in Figure 2G, the higher contents of N-5 and N-6 in NDPCs can modulate the electron cloud distribution around carbon atoms, thereby generating more active sites. These active sites not only facilitate electron transport by acting as conductive pathways but boost the conductivity of the carbon matrix. Furthermore, the enhanced interfacial dynamics between carbon matrices and ionic species enable superior electrolyte adsorption at electrode surfaces, leading to optimized charge storage through improved electrical double-layer formation at the solid-liquid interface.
Based on the above analysis, a mechanistic model for co-chemical welding is proposed [Scheme 2]. CDs, which possess abundant edge sites and high oxygen content, can interact with melamine molecules via hydrogen-bond-induced electrostatic forces. During the co-welding process, melamine decomposes and partially integrates around the highly conjugated structural units of CDs, exposing rich edge sites and defects, thereby forming a unique CDEA structure. Consequently, after co-chemical welding, the structural units primarily serve as conductive pathways, while the edge sites and pores contribute to ion adsorption.
Electrochemical performances of NDPCs in a three-electrode system
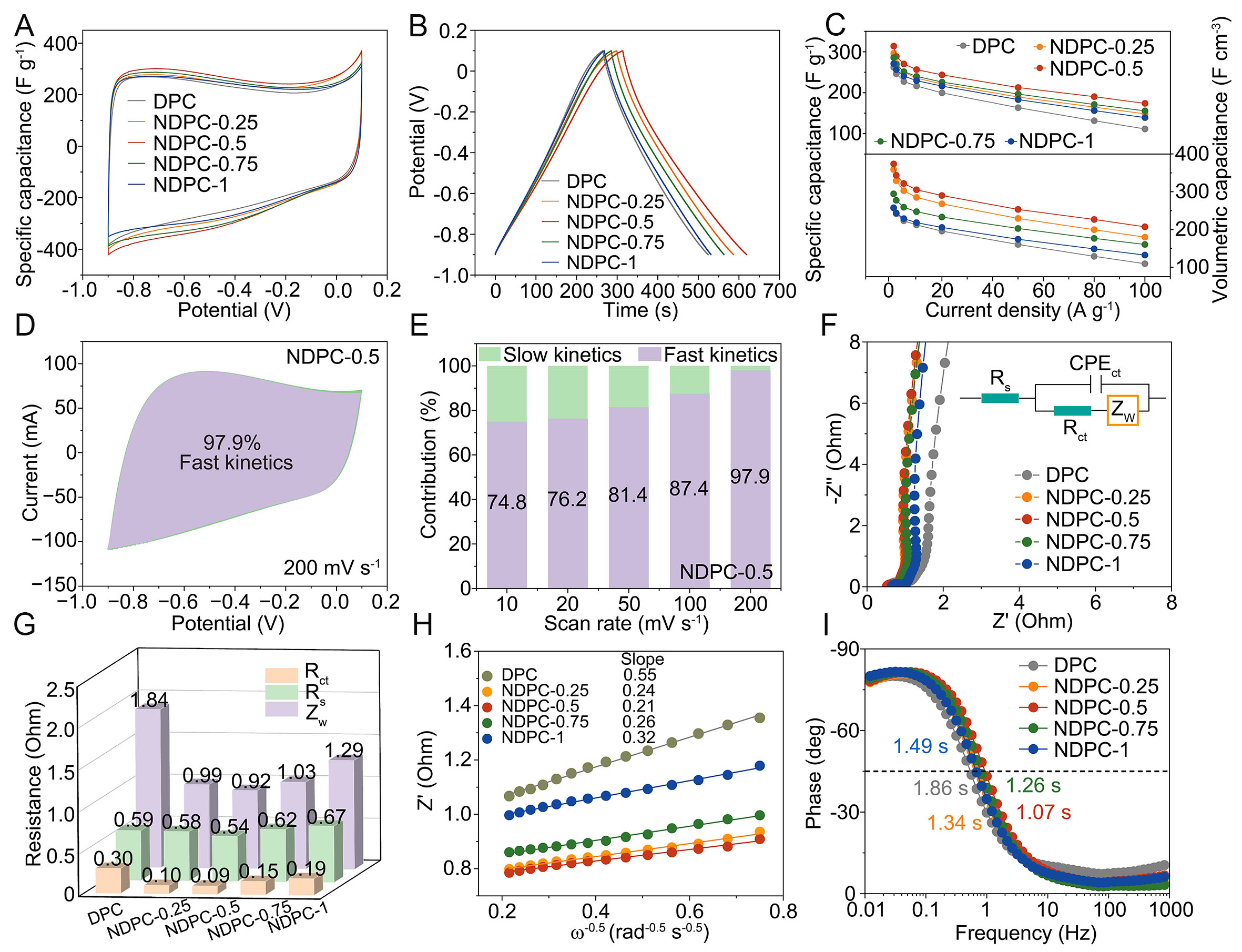
To evaluate the electrochemical energy storage performance of NDPCs, we performed systematic tests using a three-electrode system with 6 M KOH electrolyte solution. In Figure 3A and B, the cyclic voltammetry (CV) curves display characteristic near-rectangular model, whereas the galvanostatic charge-discharge (GCD) curves show slightly deformed symmetrical triangles. These observations suggest that the charge storage dynamics in NDPCs are primarily governed by electric double-layer capacitive behavior. Additionally, the introduction of heteroatoms into the material structure significantly boosts its pseudocapacitive performance. In Figure 3C, electrochemical characterization reveals that NDPC-0.5 exhibits a specific capacitance of 314 F g-1 (gravimetric) and 373.6 F cm-3 (volumetric) under 1 A g-1 current density, while retaining 61% of its initial capacitance when the current density increases to 80 A g-1. Remarkably, the material displays a substantial CV curve area even at elevated scan rates, demonstrating superior charge-discharge reversibility as evidenced in Supplementary Figure 7. This enhanced capacitive performance is primarily attributed to the synergistic effects of heteroatom doping and optimized hierarchical porous architecture. The capacitive contribution of NDPC-0.5 was further quantified using
Figure 3. Electrochemical performances of NDPCs measured by three-electrode system: (A) CV curves at 10 mV s-1, (B) GCD curves at
We conducted an analysis of the electrochemical kinetics of NDPCs using electrochemical impedance spectroscopy (EIS), as depicted in Figure 3F. The Nyquist diagram reveals characteristic capacitive features: a diminished semicircular arc in high-frequency zones transitioning to a sloped linear segment at lower frequencies. Through equivalent circuit modeling analysis, the derived series resistance values (Rs) obtained from Z′-axis intercepts demonstrate variations: NDPC-0.5 exhibits 0.54 Ω, while DPC, NDPC-0.25, NDPC-0.75, and NDPC-1 show respective values of 0.59, 0.58, 0.62, and 0.67 Ω. As illustrated in Figure 3G, these characteristics are distinctly visible. The quasi-vertical slope observed in the low-frequency range confirms the ideal capacitive behavior of NDPC-0.5, while the high-frequency semicircle reveals minimal charge transfer resistance (Rct = 0.09 Ω), highlighting efficient ion transport capabilities. Figure 3H demonstrates that NDPC-0.5 achieves the lowest Warburg diffusion coefficient (σ), indicating the hierarchical porosity facilitates rapid electrolyte penetration and ionic mobility. In contrast to DPC (1.86 s), which shows a quick electrochemical response of NDPC-0.5, bode plots [Figure 3I] show that the important relaxation time constant of NDPC-0.5 is 1.07 s. The enhanced electrochemical performance of NDPC-0.5 can be ascribed to the development of an appropriate micro-mesoporous structure, an elevated SSA, and a high oxygen content within the material. These characteristics result from optimal nitrogen doping and KOH etching, enabling efficient ion and electron transfer capabilities.
Electrochemical performances of NDPCs in symmetrical supercapacitors
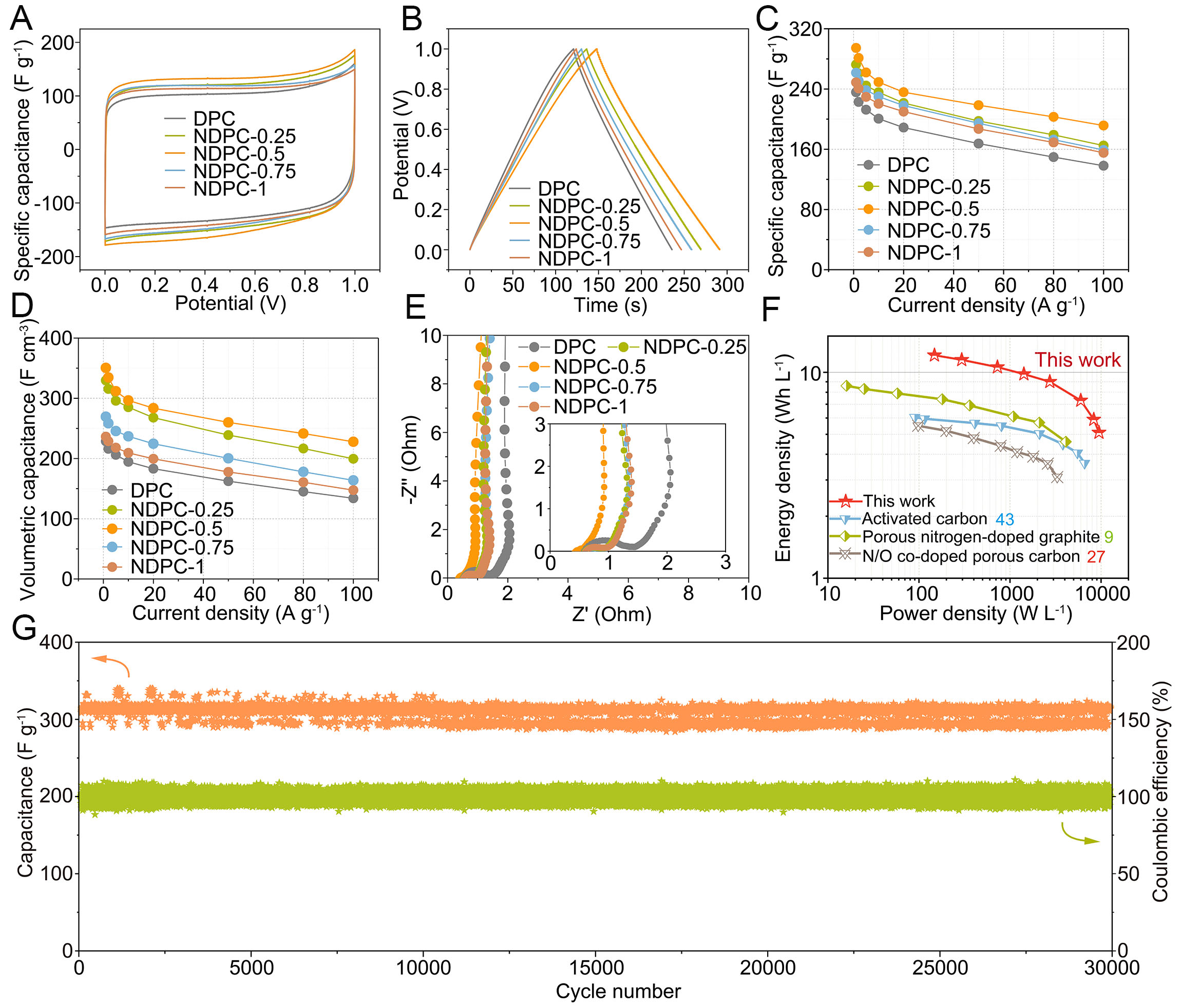
To evaluate application potential of the NDPC, symmetric supercapacitor devices were fabricated. CV measurements revealed near-rectangular characteristics, complemented by GCD curves exhibiting balanced triangular charge-discharge profiles [Figure 4A and B]. Among these, the NDPC-0.5//NDPC-0.5 configuration demonstrated the largest area, indicating excellent performance. When subjected to optimized operational parameters [Supplementary Figure 8], the NDPC-0.5 electrode maintains its characteristic rectangular morphology even under elevated current densities, demonstrating robust electrochemical stability. At elevated scanning rates (200 mV s-1), the material maintains exceptional stability, underscoring its pronounced pseudocapacitive nature and swift energy storage dynamics. Figure 4C and D illustrates the rate performance of both gravimetric and volumetric capacitance for NDPCs at various current densities. Benefiting from optimized pore architecture and numerous reactive centers, NDPC-0.5 exhibits superior charge storage capabilities, reaching notable capacitance values of 294 F g-1 (350.6 F cm-3) at 1 A g-1 and retaining 203 F g-1 (241.6 F cm-3) even under extreme current density of 80 A g-1 (retention rate is 69%). The voltage drop (IR drop) derived from the GCD curves is merely 0.14 V at
Figure 4. Electrochemical performances of the NDPCs by the two-electrode system: (A) CV curves at 10 mV s-1, (B) GCD curves at
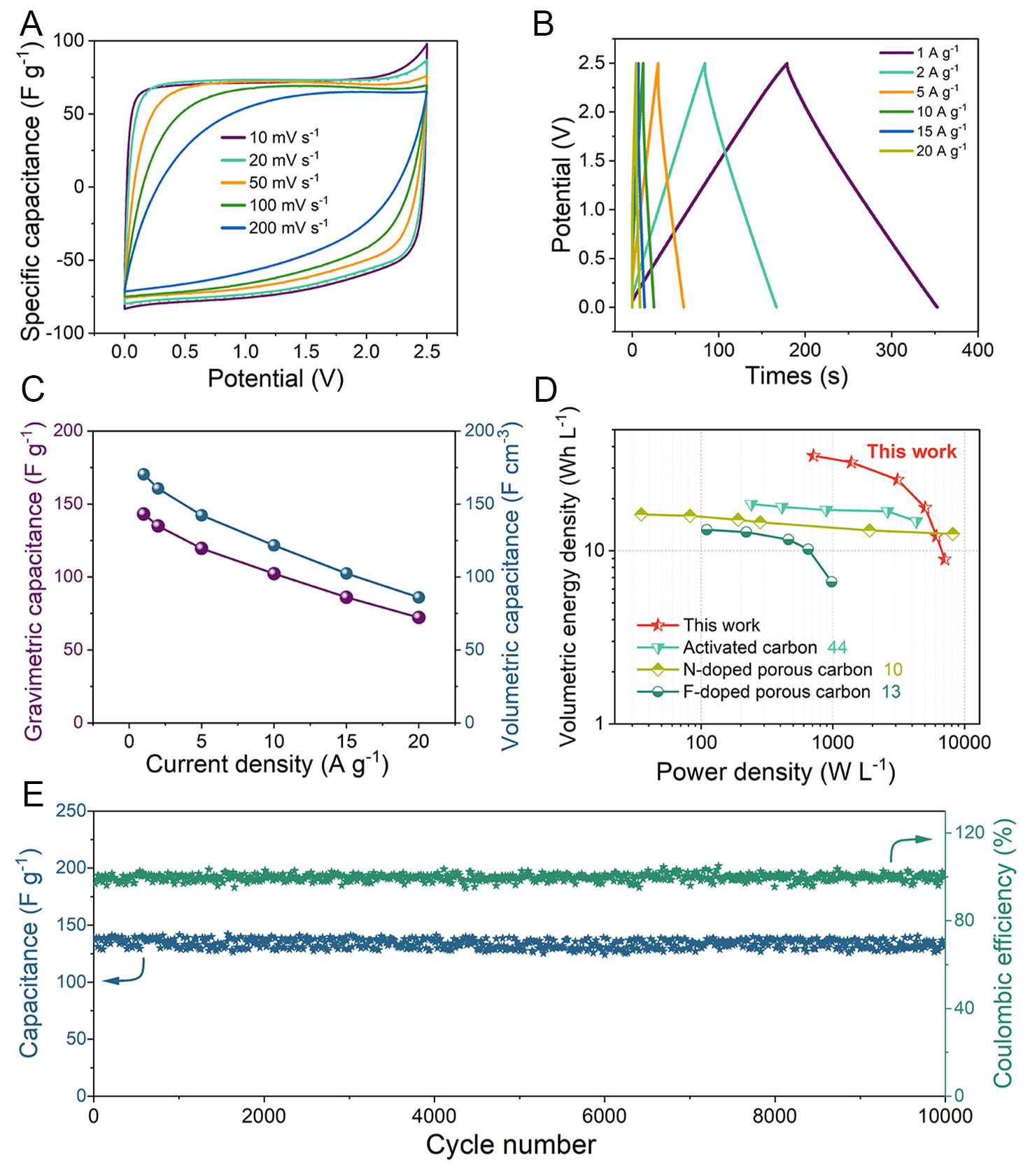
Considering the crucial role of voltage window in governing energy density through electrolyte selection, electrochemical evaluations were conducted on NDPC-0.5 using 1 M TEABF4/PC organic electrolyte to enhance energy storage capacity. Figure 5A and B exhibits well-defined rectangular and triangular shapes, suggesting superior capacitor characteristics. The NDPC-0.5//NDPC-0.5 symmetric supercapacitor demonstrates gravimetric and volumetric capacitances of 143.2 F g-1 (170.4 F cm-3) under 1 A g-1 current density, maintaining 72.3 F g-1 (85.9 F cm-3) at elevated 20 A g-1 [Figure 5C]. This electrochemical device exhibits remarkable energy storage characteristics, delivering volumetric and gravimetric energy densities of 35.3 Wh L-1 and 29.7 Wh kg-1, respectively, when operating at 711.1 W L-1 and 597.3 W kg-1 power densities, surpassing the values reported for recently developed carbon-based symmetric supercapacitors employing organic electrolytes [Figure 5D][10,13,44]. Furthermore, NDPC-0.5//NDPC-0.5 exhibits remarkable cycling stability in the organic electrolyte, maintaining a capacity retention rate of 98.7% after 10,000 cycles at
Theoretical calculation
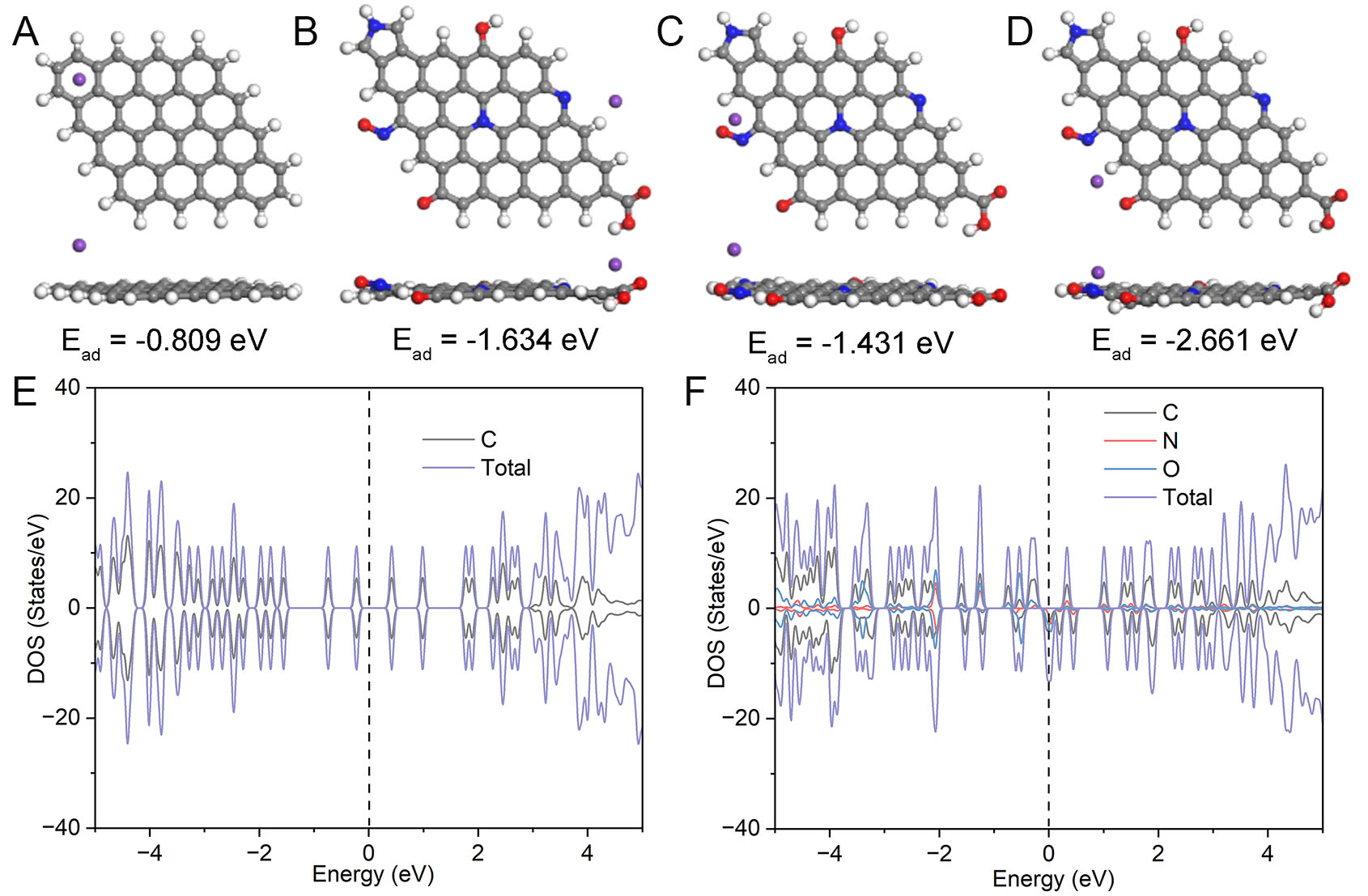
To further investigate the influence of N/O heteroatoms on the K+ adsorption capacity of carbon matrices, we performed a systematic analysis using DFT calculations. As illustrated in Figure 6A-D, the K+ adsorption energy for the pristine carbon model is -0.809 eV, whereas the K+ adsorption energies at various active sites in the N/O-doped carbon models are -1.634 eV, -1.431 eV, and -2.661 eV, respectively. In contrast, the K+ adsorption capacity of N/O-doped carbon models is significantly improved, mainly due to the introduction of N/O heteroatoms. These heteroatoms alter the electronic distribution within the carbon matrix and enhance the charge density around the adsorption sites, thus creating more active sites with appropriate adsorption strengths for K+. This also reinforces the electrostatic interaction and chemical adsorption between K+ and the carbon matrix. Furthermore, by analyzing the density of state (DOS) plots in
Figure 6. DFT calculations: (A-D) The theoretical calculation models with top- (upper) and side-view (lower) for K+ absorbed on different models as well as corresponding Ead (purple, grey, blue, red, and white colors indicate K, C, N, O, and H atoms). (E and F) Densities of state (DOS) of pure C and N/O co-doped C.
CONCLUSION
In this study, we have synthesized NDPCs with a novel CDEA structure by utilizing CLR-derived CDs and melamine. The approach not only enhances the bulk density of the material to 1.19 g cm-3 but also creates a three-dimensional conductive network that significantly reduces electrode impedance. The co-chemical welding and N/O co-doping strategy effectively increases the material’s density while introducing additional nitrogen-doped active sites, which are crucial for improving ion adsorption capacity. Our experimental results demonstrate remarkable enhancements in both volumetric and gravimetric specific capacitances, with the NDPCs outperforming undoped DPC. Furthermore, DFT calculations validate the synergistic effect of N/O co-doping in reducing K+ adsorption energy, thereby enhancing ion adsorption capabilities. This work offers a promising and innovative strategy for the development of high-performance supercapacitor electrode materials, leveraging the unique properties of NDPCs to advance the field of energy storage devices.
DECLARATIONS
Authors’ contributions
Conceptualization, data curation, methodology, visualization, writing - original draft: Wang, Z.; Chen, Y.
Formal analysis: Chen, Y.; Wang, Z.; Mei, X.
Funding acquisition: Xie, H.; Li, Y.
Investigation: Chen, Y.; Wang, Z.; Xie, H.; Li, Y.
Supervision: Li, Y.
Writing - review and editing: Wang, Q.; Xie, H.; Li, Y.
Availability of data and materials
The raw data supporting the conclusions of this article will be made available by the authors on request.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (Nos. 52100166 and 22306023) and the Key Research and Development Project of Huzhou City (No. 2023ZD2034).
Conflicts of interest
Wang, Q. is affiliated with Huadian Electric Power Research Institute Co., Ltd, , while the other authors have declared that they have no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Wang, T.; Pan, R.; Martins, M. L.; et al. Machine-learning-assisted material discovery of oxygen-rich highly porous carbon active materials for aqueous supercapacitors. Nat. Commun. 2023, 14, 40282.
2. Wang, Z.; Liu, Y.; Guo, Y.; et al. Coral polyp and spine dual-inspired gradient hierarchical architecture for ultrahigh-rate and long-life sodium storage. Adv. Funct. Mater. 2024, 34, 2402178.
3. Ma, C.; Zhu, B.; Wang, Y.; et al. Porous carbon nanosheets integrated with graphene-wrapped CoO and CoNx as efficient bifunctional oxygen electrocatalysts for rechargeable zinc-air batteries. J. Colloid. Interface. Sci. 2025, 685, 793-803.
4. Mo, T.; Wang, Z.; Zeng, L.; et al. Energy storage mechanism in supercapacitors with porous graphdiynes: effects of pore topology and electrode metallicity. Adv. Mater. 2023, 35, 2301118.
5. Chang, L.; Chen, S.; Fei, Y.; Stacchiola, D. J.; Hu, Y. H. Superstructured NiMoO4@CoMoO4 core-shell nanofibers for supercapacitors with ultrahigh areal capacitance. Proc. Natl. Acad. Sci. USA. 2023, 120, e2219950120.
6. Li, X.; Zheng, Q.; Li, C.; et al. Bubble up induced graphene microspheres for engineering capacitive energy storage. Adv. Energy. Mater. 2023, 13, 2203761.
7. Liu, Q.; Wu, D.; Wang, T.; Wang, C.; Jia, D. Pre-oxidating and pre-carbonizing to regulate the composition and structure of coal tar pitch: the fabrication of porous carbon for supercapacitor applications. Adv. Funct. Mater. 2024, 34, 2400556.
8. He, X.; Ma, H.; Wang, J.; Xie, Y.; Xiao, N.; Qiu, J. Porous carbon nanosheets from coal tar for high-performance supercapacitors. J. Power. Sources. 2017, 357, 41-6.
9. Zhang, Z.; Gao, Z.; Zhang, Y.; et al. Hierarchical porous nitrogen-doped graphite from tissue paper as efficient electrode material for symmetric supercapacitor. J. Power. Sources. 2021, 492, 229670.
10. Cheng, J.; Lu, Z.; Zhao, X.; Chen, X.; Liu, Y. Green needle coke-derived porous carbon for high-performance symmetric supercapacitor. J. Power. Sources. 2021, 494, 229770.
11. Liu, X.; Lyu, D.; Merlet, C.; et al. Structural disorder determines capacitance in nanoporous carbons. Science 2024, 384, 321-5.
12. Wang, C.; Wu, D.; Wang, H.; Gao, Z.; Xu, F.; Jiang, K. A green and scalable route to yield porous carbon sheets from biomass for supercapacitors with high capacity. J. Mater. Chem. A. 2018, 6, 1244-54.
13. Zhou, H.; Peng, Y.; Wu, H. B.; et al. Fluorine-rich nanoporous carbon with enhanced surface affinity in organic electrolyte for high-performance supercapacitors. Nano. Energy. 2016, 21, 80-9.
14. Yang, X.; Zhao, S.; Zhang, Z.; et al. Pore structure regulation of hierarchical porous carbon derived from coal tar pitch via pre-oxidation strategy for high-performance supercapacitor. J. Colloid. Interface. Sci. 2022, 614, 298-309.
15. Che, X.; Yang, J.; Liu, S.; Wang, M.; He, S.; Qiu, J. Multilayer-dense porous carbon nanosheets with high volumetric capacitance for supercapacitors. Ind. Eng. Chem. Res. 2022, 61, 8908-17.
16. Zhang, S.; Zhu, J.; Qing, Y.; et al. Ultramicroporous carbons puzzled by graphene quantum dots: integrated high gravimetric, volumetric, and areal capacitances for supercapacitors. Adv. Funct. Mater. 2018, 28, 1805898.
17. Li, Q.; Jiang, Y.; Jiang, Z.; et al. Ultrafast pore-tailoring of dense microporous carbon for high volumetric performance supercapacitors in organic electrolyte. Carbon 2022, 191, 19-27.
18. Jiang, Y.; Li, J.; Jiang, Z.; et al. Large-surface-area activated carbon with high density by electrostatic densification for supercapacitor electrodes. Carbon 2021, 175, 281-8.
19. Zheng, Y.; Chen, K.; Jiang, K.; Zhang, F.; Zhu, G.; Xu, H. Progress of synthetic strategies and properties of heteroatoms-doped (N, P, S, O) carbon materials for supercapacitors. J. Energy. Storage. 2022, 56, 105995.
20. Wang, Q.; Su, J.; Chen, H.; et al. Highly conductive nitrogen-doped sp2/sp3 hybrid carbon as a conductor-free charge storage host. Adv. Funct. Mater. 2022, 32, 2209201.
21. Srinivasan, S. B.; Devendiran, S.; Savunthari, K. V.; Arumugam, P.; Mukerjee, S. Insights into multifarious heteroatom-doped/enriched carbon-based materials and their composites: Synthesis and Supercapacitor applications - a crucial review. Prog. Mater. Sci. 2025, 153, 101470.
22. Lou, G.; Pei, G.; Wu, Y.; et al. Combustion conversion of wood to N, O co-doped 2D carbon nanosheets for zinc-ion hybrid supercapacitors. Chem. Eng. J. 2021, 413, 127502.
23. Ghosh, S.; Barg, S.; Jeong, S. M.; Ostrikov, K. Heteroatom-doped and oxygen-functionalized nanocarbons for high-performance supercapacitors. Adv. Energy. Mater. 2020, 10, 2001239.
24. Zhang, N.; Liu, F.; Xu, S.; Wang, F.; Yu, Q.; Liu, L. Nitrogen-phosphorus co-doped hollow carbon microspheres with hierarchical micro-meso-macroporous shells as efficient electrodes for supercapacitors. J. Mater. Chem. A. 2017, 5, 22631-40.
25. Wu, Z.; Winter, A.; Chen, L.; et al. Three-dimensional nitrogen and boron co-doped graphene for high-performance all-solid-state supercapacitors. Adv. Mater. 2012, 24, 5130-5.
26. Liu, H.; Zhu, S.; Zhang, Y.; et al. Unveiling superior capacitive behaviors of one-pot molten salt-engineered B, N Co-doped porous carbon sheets. Small 2023, 19, 2204119.
27. Gao, H.; Zhang, D.; Zhou, H.; et al. Boosting gravimetric and volumetric energy density of supercapacitors by 3D pomegranate-like porous carbon structure design. Appl. Surf. Sci. 2020, 534, 147613.
28. Li, H.; Tao, Y.; Zheng, X.; et al. Ultra-thick graphene bulk supercapacitor electrodes for compact energy storage. Energy. Environ. Sci. 2016, 9, 3135-42.
29. Murali, S.; Quarles, N.; Zhang, L. L.; et al. Volumetric capacitance of compressed activated microwave-expanded graphite oxide (a-MEGO) electrodes. Nano. Energy. 2013, 2, 764-8.
30. Chen, Y.; Qin, F.; Wang, Z.; et al. Dense porous carbon from chemical welding the oxidized coal liquefaction residue for enhanced volumetric performance supercapacitors. J. Energy. Storage. 2023, 72, 108542.
31. Yu, X.; Lu, J.; Zhan, C.; et al. Synthesis of activated carbon nanospheres with hierarchical porous structure for high volumetric performance supercapacitors. Electrochim. Acta. 2015, 182, 908-16.
32. Li, Q.; Zhang, S.; Jiang, Y.; et al. Preparation of high density activated carbon by mechanical compression of precursors for compact capacitive energy storage. Acta. Phys. Chim. Sin. 2025, 41, 100028.
33. Li, Y.; Chen, M.; Liu, B.; Zhang, Y.; Liang, X.; Xia, X. Heteroatom doping: an effective way to boost sodium ion storage. Adv. Energy. Mater. 2020, 10, 2000927.
34. Chen, C.; Huang, Y.; Meng, Z.; Xu, Z.; Liu, P.; Li, T. Multi-heteroatom doped porous carbon derived from insect feces for capacitance-enhanced sodium-ion storage. J. Energy. Chem. 2021, 54, 482-92.
35. Zhao, J.; Li, Y.; Wang, G.; et al. Enabling high-volumetric-energy-density supercapacitors: designing open, low-tortuosity heteroatom-doped porous carbon-tube bundle electrodes. J. Mater. Chem. A. 2017, 5, 23085-93.
36. Yan, L.; Li, D.; Yan, T.; et al. N,P,S-codoped hierarchically porous carbon spheres with well-balanced gravimetric/volumetric capacitance for supercapacitors. ACS. Sustain. Chem. Eng. 2018, 6, 5265-72.
37. Chen, C.; Zhao, M.; Cai, Y.; et al. Scalable synthesis of strutted nitrogen doped hierarchical porous carbon nanosheets for supercapacitors with both high gravimetric and volumetric performances. Carbon 2021, 179, 458-68.
38. Yu, W.; Meng, Y.; Gong, J.; et al. N/O/P Co-doped highly microporous carbons with optimized volumetric and gravimetric supercapacitive performance. ACS. Appl. Energy. Mater. 2025, 8, 801-9.
39. Liang, K.; Zou, K.; Liu, J.; Deng, Y.; Chen, G. An interlayer spacing-anion matching guideline for high-performance N-doped porous carbon cathode. ACS. Energy. Lett. 2023, 8, 3204-13.
40. Wu, X.; Liu, J.; Wang, Y.; Zhao, Y.; Li, G.; Zhang, G. Fabrication of porous carbon nanosheets via urea-promoted activation of potassium citrate for enhanced supercapacitor performance. Chem. Eng. J. 2025, 512, 162487.
41. Xu, Z.; Dou, R.; Tan, Y.; et al. Facile and green preparation of carbonaceous material-based wood bio-adhesives using hydrochar from hydrothermal carbonization of glucose with or without acrylic acid/acrylamide. Int. J. Adhes. Adhes. 2025, 136, 103851.
42. Wu, D.; Sun, F.; Wang, H.; et al. Mechanochemical process enhancing pore reconstruction for dense energy storage of carbon-based supercapacitors. Energy. Mater. 2025, 5, 500055.
43. Shen, C.; Li, R.; Yan, L.; et al. Rational design of activated carbon nitride materials for symmetric supercapacitor applications. Appl. Surf. Sci. 2018, 455, 841-8.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.