The relationship between emerging contaminants and gestational diabetes mellitus: a review
Abstract
Emerging contaminants (ECs) have garnered growing attention as potential contributors to adverse metabolic outcomes during pregnancy, particularly gestational diabetes mellitus (GDM). Despite increasing recognition of their endocrine-disrupting capabilities, the precise relationship between EC exposure and glucose dysregulation in the gestational context remains inadequately characterized. The scarcity of longitudinal human studies, along with limited mechanistic elucidation, highlights a critical gap in understanding how these ubiquitous environmental pollutants may perturb maternal metabolic homeostasis. This review consolidates current epidemiological evidence linking key classes of ECs, including per- and polyfluoroalkyl substances (PFAS), polychlorinated biphenyls (PCBs), phthalates (PAEs), bisphenols, organochlorine pesticides (OCPs), parabens, and alkylphenols, with GDM risk and impaired glycemic control. Parallel examination of in vivo and in vitro studies reveals plausible biological mechanisms, including oxidative stress, mitochondrial dysfunction, inflammatory signaling, and insulin resistance (IR), through which these compounds may mediate their effects. By integrating data across human and experimental research domains, this review underscores the urgent need for high-resolution exposure assessments, mixture toxicity frameworks, and mechanistic validation. Such insight is essential for advancing etiological understanding, informing regulatory action, and guiding preventive strategies to mitigate the impact of environmental exposures on maternal-fetal metabolic health.
Keywords
INTRODUCTION
Gestational diabetes mellitus (GDM) is a form of glucose intolerance first identified during pregnancy and is among the most common complications affecting maternal health (see Supplementary Table 1 for abbreviations)[1]. According to the 2024 IDF Diabetes Atlas, hyperglycemia affects nearly one in five pregnancies worldwide, and GDM increases the risk of adverse outcomes such as preterm birth, macrosomia, and cesarean delivery[2]. Furthermore, it poses long-term health risks, including a higher likelihood of developing type 2 diabetes (T2DM) and metabolic disorders in both mothers and their children[3]. Known risk factors for GDM include advanced maternal age, obesity, family history of diabetes, and excessive gestational weight gain. However, emerging evidence suggests that environmental exposures, particularly to emerging contaminants (ECs), may also contribute to its development[4-11].
ECs refer to chemicals or materials that pose a potential or recognized threat to human health or the environment[12]. These substances are typically not regulated or lack established health standards, and their presence is increasing in various environmental matrices[12,13]. ECs include persistent organic pollutants, pharmaceuticals and their metabolites, personal care products, nanomaterials, and microplastics[14]. Due to their resistance to degradation, many ECs persist in water, soil, and air, and are difficult to remove through conventional treatment methods. For example, a study has detected pharmaceuticals and personal care products (PPCPs) in surface water, seawater, groundwater, and wastewater treatment plants in the Middle East and North Africa region[15]. These contaminants enter the human body primarily through ingestion, inhalation, or skin contact[15]. Despite often being present at low concentrations, the biotoxicity, environmental persistence, and bioaccumulation of these substances raise significant concerns.
Recent studies have started to explore the relationship between EC exposure and the development of GDM. However, most of these studies have focused on individual pollutants or their congeners, often within the context of either epidemiological studies or animal models. A comprehensive review encompassing a broad spectrum of ECs, integrating both epidemiological findings and experimental data, is currently lacking.
This review aims to provide a systematic summary of epidemiological studies on the impact of seven major types of ECs: PFAS, PCBs, PAEs, bisphenols, OCPs, parabens, and alkylphenols, examining their effects on glucose metabolism, insulin resistance (IR), and the development of GDM. In cases where relevant, insights from experimental studies are included to illuminate potential mechanisms of action. By integrating diverse evidence, this review contributes to a holistic understanding of the environmental factors influencing GDM and provides a scientific foundation for future research and policy development.
PER- AND POLYFLUOROALKYL SUBSTANCES
Per- and polyfluoroalkyl substances (PFAS) are a group of synthetic chemicals, including perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), perfluorononanoic acid (PFNA), perfluorohexanesulfonic acid (PFHxS), and perfluorodecanoic acid (PFDA)[16]. These substances are widely used in consumer products such as carpets, textiles, firefighting foams, sunscreens, and cosmetics[17]. They are also applied as coating agents in paper and cardboard packaging materials[17]. Due to the strong stability of the carbon-fluorine bond, PFAS exhibit exceptional chemical and thermal stability[18]. As a result, they persist in the environment and are frequently detected in multiple environmental matrices, including water, soil, and vegetation[19]. PFAS are also commonly found in human samples, such as urine, blood, breast milk, amniotic fluid, and other tissues[20,21]. Human exposure to PFAS occurs primarily through ingestion of contaminated food and drinking water[22]. Given their widespread use and persistence, PFAS are increasingly recognized as significant environmental contaminants. However, the full extent of their health impacts, particularly in relation to metabolic disorders such as GDM, remains an area of ongoing research.
Epidemiological studies
Epidemiological evidence on the association between PFAS exposure and the risk of GDM remains inconsistent; relevant studies are summarized in Table 1[5,9,23-44]. In a cross-sectional study in China, researchers measured the concentrations of 13 PFAS in umbilical cord blood and found that exposure to several PFAS, including perfluoroheptanoic acid (PFHpA), PFNA, and perfluorobutanesulfonic acid (PFBS), was significantly associated with elevated mid-pregnancy blood glucose levels[23]. Similarly, a study conducted in Hong Kong identified PFOS and PFOA as the predominant PFAS compounds, with concentrations in late pregnancy higher than those reported in other studies[5]. Both PFAS mixtures and individual compounds were significantly associated with elevated maternal levels of HbA1c and 2-hour plasma glucose (2h-PG)[5].
Summary of epidemiological evidence on the association between PFAS exposure and GDM
| Study type | Year | Biological sample | Population | Findings (GM/Med, ng/mL) | Author | |||
| Analyte | Matrix | Gestational week | Country | Size | ||||
| Cross-sectional | 2020 | PFAS | Umbilical cord blood | At delivery | China | 874 | Positive association with increased blood glucose in 2nd trimester: PFHpA [0.04], PFNA [0.27], and PFBS [0.04]a | Li et al.[23] |
| Cross-sectional | 2024 | PFAS | Serum | 24-32 weeks of gestation | China | 1,601 | PFAS mixture and its individual components were associated with higher maternal HbA1c and 2h-PG | Yang et al.[5] |
| Case-control | 2018 | PFAS | Serum | 1-2 days before delivery | China | 252 | Positive association with postpartum fasting glucose: 1m-PFOS [0.14 vs. 0.14], 3m + 4m-PFOS [0.44 vs. 0.42], 5m-PFOS [0.36 vs. 0.36], PFHxS [0.48 vs. 0.47]b | Wang et al.[24] |
| Case-control | 2020 | PFAS | Serum | 16-20 weeks of gestation | China | 495 | Positive association between PFBS [0.17 vs. 0.13], PFDoA [0.19 vs. 0.08] and GDMb | Xu et al.[25] |
| Case-control | 2021 | PFAS | Serum | 1st trimester | China | 231 | Higher PFHxA [0.025 vs. 0.015] levels in GDM casesc | Liu et al.[26] |
| Case-control | 2022 | PFAS | Serum | 1-2 days before the delivery | China | 340 | Positive association with GDM: PFOA [22.6 vs. 2.32], PFOS [5.98 vs. 3.29], PFUnDA [2.90 vs. 1.06], PFDoA [0.250 vs. 0.175] and 6:2Cl-PFESA [5.30 vs. 2.38] Negative association with GDM: 4:2FTS [0.150 vs. 0.250], 6:2FTS [0.050 vs. 0.050], PFHxS [0.150 vs. 1.03] and ADONA [0.175 vs. 0.150]b | Xu et al.[27] |
| Case-control | 2023 | PFAS | Serum | 1st and 2nd trimester | USA | 128 | Positive association with GDM in 1st and 2nd trimester: PFDA [ 0.09; 0.07], PFNA [0.39; 0.35], and PFOA [0.71; 0.69]b | Peterson et al.[28] |
| Case-control | 2023 | PFAS | Serum | 1st trimester | China | 590 | Positive association between PFOA [10.3 vs. 9.52], PFHpS [0.09 vs. 0.08] and GDM A nonlinear association between 6:2Cl-PFESA [3.93 vs. 3.68] and GDMb | Zang et al.[29] |
| Case-control | 2023 | PFAS | Serum | 1st trimester | China | 204 | Positive association with GDM: PFOA [5.22 vs. 5.03], PFNA [0.48 vs. 0.48], PFHxS [0.45 vs. 0.43] and 6:2 Cl - PFESA [2.58 vs. 2.42]b | Zhang et al.[30] |
| Cohort | 2015 | PFAS | Serum | NA | USA | 258 | Positive association between PFOA [3.94 vs. 3.07] and GDMa | Zhang et al.[31] |
| Cohort | 2016 | PFAS | Plasma | 1st trimester | Canada | 1,274 | Positive association between PFHxS [1.00 vs. 1.02] and IGTa | Shapiro et al.[32] |
| Cohort | 2017 | PFAS | Plasma | 1st trimester | Spain | 1,240 | Positive association between PFOS [5.77], PFHxS [0.55] and IGTa | Matilla-Santander et al.[33] |
| Cohort | 2017 | PFAS | Serum | 20-34 weeks of gestation | USA | 628 | Negative association with maternal fasting glucose: PFOA [1.04], PFNA [0.39], PFDeA [0.14], PFHxS [0.75]a | Starling et al.[34] |
| Cohort | 2017 | PFAS | Serum | 34 weeks of gestation | Denmark | 604 | No association | Valvi et al.[35] |
| Cohort | 2018 | PFAS | Serum | 11 gestational week | Denmark | 318 | Positive association between PFHxS [0.30] and increased fasting glucose, fasting insulin and HOMA-IR Positive association between PFNA [0.66] and higher fasting insulin and HOMA-%βb | Jensen et al.[36] |
| Cohort | 2018 | PFAS | Serum | 1st trimester | China | 385 | Positive association between PFOA [7.3] and FIns, HOMA-IR, 1h-PG, 2h-PG Negative association between PFOS [5.4] and averaged FBG and OGTT blood glucoseb | Wang et al.[37] |
| Cohort | 2019 | PFAS | Plasma | 8–13 weeks of gestation | USA | 2,292 | Positive association with GDM in T2DM family history: PFNA [0.80], PFOA [1.99], PFHpA [0.08], PFDoDA [0.06]a | Rahman et al.[9] |
| Cohort | 2020 | PFAS | Plasma | 1st trimester | USA | 1,540 | Positive association between PFOS [25.5] and 1h-GCT levels A nonlinear association between MeFOSAA [1.9] and 1h-GCT levelsa | Preston et al.[38] |
| Cohort | 2020 | PFAS | Plasma | 1st trimester | China | 981 | Positive association with 1h-PG: PFNA [1.8], PFDA [2.0], PFUdA [1.6], PFDoA [0.1]a | Ren et al.[39] |
| Cohort | 2021 | PFAS | Serum | Late 1st or 2nd trimester | USA | 95 | All PFAS [5.81] was negatively associated with fasting glucose, fasting insulin, and HOMA-IRa | Mehta et al.[40] |
| Cohort | 2021 | PFAS | Serum | 16 and 26 weeks of gestation | USA | 388 | No association | Vuong et al.[41] |
| Cohort | 2021 | PFAS | Plasma | 1st trimester | China | 2,747 | Positive association between PFBS [0.045 vs. 0.035], PFHpA [0.061 vs. 0.054] and GDM; Positive association with 1h-PG and 2h-PG: PFOS [9.41 vs. 9.40], PFNA [1.61 vs. 1.65], PFHxS [0.54 vs. 0.53], PFHpA [0. 061 vs. 0.054]b | Yu et al.[42] |
| Cohort | 2023 | PFAS | Plasma | 1st trimester | China | 1,405 | Higher PFOA and PFHxS and lower PFUA in high FPG group | Wang et al.[43] |
| Cohort | 2024 | PFAS | Serum | Median 17 weeks gestation | USA | 452 | All PFAS (except Me-PFOSA-AcOH) were negatively associated with insulin, HOMA-IR, and leptin | Cinzori et al.[44] |
A total of seven case-control studies were reviewed, with six consistently finding a positive association between PFAS exposure and the risk of GDM[24-30]. For instance, a study by Xu et al. enrolled 165 GDM cases and 330 controls and found that elevated levels of PFBS and perfluorododecanoic acid (PFDoA) in early pregnancy were significantly associated with an increased risk of GDM[25]. In a study analyzing maternal serum samples collected 1-2 days before delivery, PFOA, PFOS, perfluoroundecanoic acid (PFUnDA), PFDoA, and 6:2 chlorinated polyfluorinated ether sulfonic acid (6:2 Cl-PFESA) were found to be positively associated with GDM risk, while 4:2 Fluorotelomer sulfonate (4:2 FTS), 6:2 Fluorotelomer sulfonate (6:2 FTS), PFHxS, and ammonium 4,8-dioxa-3H-perfluorononanoate (ADONA) showed negative associations[27]. Similarly, case-control studies conducted in other regions reported comparable findings. A study conducted in the United States reported significant positive associations between PFOA, PFOS, and other PFAS compounds in early and mid-pregnancy and the risk of GDM[28].
Despite the heterogeneity in findings, the majority of cohort studies have reported a positive association between PFAS exposure and the risk of GDM[9,31-44]. A study by Rahman et al. reported that, among pregnant women with a family history of T2DM, higher maternal plasma levels of PFNA, PFOA, PFHpA, and PFDoDA in early pregnancy were positively associated with the risk of GDM[9]. Similarly, Ren et al. investigated the relationship between maternal plasma concentrations of PFAS in early pregnancy and blood glucose levels, and found that PFNA, PFDA, perfluoroundecanoic acid (PFUdA), and PFDoA were positively associated with 1-hour plasma glucose (1h-PG) levels[39]. However, some studies reported inverse associations, such as the one conducted by Mehta et al., which found that PFAS exposure was inversely associated with fasting glucose, fasting insulin, and Homeostasis Model Assessment of Insulin Resistance (HOMA-IR)[40].
In summary, while the epidemiological evidence remains inconclusive, the majority of studies suggest that PFAS exposure may be a potential environmental risk factor for GDM. Further prospective studies and mechanistic research are necessary to validate these associations and clarify the underlying biological mechanisms.
Experimental studies and possible mechanisms
Emerging experimental evidence indicates that PFAS, particularly PFOS and PFBS, may impair pancreatic β-cell function and insulin secretion through oxidative stress, mitochondrial dysfunction, and key signaling pathway alterations. In vivo and in vitro studies showed that PFOS exposure reduced pancreas weight, islet size, and serum insulin levels in male mice, and decreased viability and glucose-stimulated insulin release of β-TC-6 cells. High-dose PFOS induced ROS accumulation, leading to apoptosis and necrosis[45]. Collectively, these findings support a potential causal link between PFOS exposure and increased diabetes risk[45]. Similarly, adult male Balb/c mice orally administered 1.25 mg/kg PFOA for 28 days exhibited elevated fasting blood glucose (FBG) levels, reduced hepatic glycogen and glucose content, and alterations in key metabolic enzyme activities such as decreased glycogen synthase activity and increased glucose-6-phosphatase activity[46]. Together, these findings suggest that PFOA may promote hepatic glycogen breakdown and elevate glucose export from the liver into the bloodstream, while also enhancing the downstream glycolytic pathway and the tricarboxylic acid cycle[46].
PFOS exposure has similarly been associated with impaired glucose metabolism, particularly during developmental stages. Pregnant CD-1 mice exposed to PFOS during gestation and lactation gave birth to offspring that exhibited glucose metabolism disturbances in early life, with later exacerbation of IR and glucose intolerance, especially when exposed to a high-fat diet[47]. These findings imply that early-life PFOS exposure may predispose offspring to long-term metabolic vulnerabilities[47].
Moreover, studies involving Sprague-Dawley rats have demonstrated that gestational exposure to low doses of PFOS induces glucolipid metabolic disorders through disruption of peroxisome proliferator-activated receptor signaling and alterations in glycerolipid and glycolysis pathways[48]. Altered gene expression and metabolite changes, including glycerol 3-phosphate and lactosylceramide, have been observed[48].
Another study in pregnant rats found that high-dose PFBS exposure (50 mg/kg bw·d) significantly reduced 1h-PG and the OGTT (Oral Glucose Tolerance Test) area under the curve, suggesting altered glucose homeostasis[49]. Integrated transcriptomics and metabolomics revealed changes in pathways related to xenobiotic metabolism, glutathione metabolism, bile acid secretion, pyruvate metabolism, and the citric acid cycle. Key differentially expressed genes (e.g., Gstm1, Gck, Ppp1r3c) and metabolites (e.g., fumaric acid, L-lactic acid) were linked to glucolipid metabolic regulation[49]. These findings indicate that gestational PFBS exposure may disrupt glucose metabolism at the molecular level, potentially increasing the risk of abnormal glucose tolerance[49].
Although the PFAS family includes many compounds such as PFNA and PFHxS, mechanistic evidence in animal experiments is currently most extensive for PFOA, PFOS, and PFBS. These studies suggest that PFAS exposure, particularly during critical developmental windows, may lead to persistent metabolic disruptions by targeting hepatic enzyme function, nuclear receptor signaling, and intermediary metabolism. Although experimental doses are higher than typical environmental exposures, they provide valuable mechanistic insights into PFAS-induced β-cell dysfunction, impaired insulin secretion, and glucose dysregulation.
POLYCHLORINATED BIPHENYLS
Polychlorinated biphenyls (PCBs) are synthetic organochlorine compounds composed of two linked benzene rings with varying chlorine substitutions[50]. Historically, PCBs were widely applied as plasticizers and in industrial coolants[50]. Due to their chemical stability, lipophilicity, and resistance to degradation, PCBs persist in the environment and bioaccumulate through the food chain[51]. Despite being banned under the United States Toxic Substances Control Act in 1979, their extensive use and environmental persistence have led to continued detection in air, soil, water, and biological matrices, including human blood and breast milk[52,53]. Human exposure occurs through multiple pathways, including diet, inhalation, dust ingestion, and dermal absorption[54]. The widespread presence and long half-life of PCBs have raised considerable public health concerns, prompting extensive research into their potential toxicological effects.
Epidemiological studies
Relatively few studies have investigated the relationship between PCBs and GDM, with existing research primarily adopting a prospective cohort design. These epidemiological studies are summarized in
Summary of epidemiological evidence on the association between PCBs, PAE exposure and GDM
| Study type | Year | Biological sample | Population | Findings (GM/Med) | Author | |||
| Analyte | Matrix | Gestational week | Country | Size | ||||
| Cross-sectional | 2020 | PCBs | Serum | Before pregnancy | USA | 254 | No association | Neblett et al.[55] |
| Case-control | 2016 | PCBs | Serum | 3rd trimester | Iran | 140 | Positive association with GDM: total PCBs [3.09 vs. 1.60 ng/g lipid], Ln PCB-187 [0.03 vs. 0 01 ng/g lipid], Ln PCB-118 [0.03 vs. 0.01 ng/g lipid] Negative association with GDM: Ln PCB-28 [0.02 vs. 0.01 ng/g lipid]a,d | Eslami et al.[57] |
| Case-control | 2018 | PCBs | Serum | 1st trimester | China | 231 | Positive association between PCB-52 [2.0 vs. 1.5 pg/mL] and GDM, all blood glucose values of OGTTb | Zhang et al.[56] |
| Case-control | 2021 | PCBs | Serum | 1st trimester | China | 231 | Higher PCB-52 [1.98 vs.1.45 pg/g], PCB-101 [1.40 vs. 0.99 pg/g] levels in GDM casesb | Liu et al.[26] |
| Case-Control | 2023 | PCBs | Serum | 1st trimester | China | 208 | Positive association between PCB-153 [0.96 vs. 0.59 ng/g lipid], ∑PCB [3.57 vs. 2.61 ng/g lipid] and GDM, 1h-PGb | Ma et al.[6] |
| Cohort | 2016 | PCBs | Serum | Before pregnancy | USA | 258 | Negative association with GDM after adjustment for total serum lipids: PCB-138 [0.03 ng/g serum], PCB-153 [0.0423 ng/g serum], PCB-156 [0.0054 ng/g serum], PCB-167 [0.0000 ng/g serum], PCB-170 [0.0127 ng/g serum], PCB-172 [0.0000 ng/g serum], PCB-178 [0.0017 ng/g serum], PCB-180 [0.0320 ng/g serum], and PCB-194 [0.0064 ng/g serum]b | Jaacks et al.[58] |
| Cohort | 2016 | PCBs | Plasma | 1st trimester | Canada | 1,274 | No association | Shapiro et al.[32] |
| Cohort | 2017 | PCBs | Serum | 1st trimester | Greece | 939 | Positive association with GDM [∑PCBs 360.1 vs. 290.8 pg/mL]a | Vafeiadi et al.[59] |
| Cohort | 2017 | PCBs | Serum | 34 weeks of gestation | Denmark | 604 | No association | Valvi et al.[35] |
| Cohort | 2019 | PCBs | Plasma | 8-13 weeks of gestation | USA | 2,292 | Positive association between PCBs with ≥ 6 chlorine atoms and GDM | Rahman et al.[9] |
| Cohort | 2021 | PCBs | Serum | Late 1st or 2nd trimester | USA | 95 | Positive association between individual PCBs and fasting insulin, HOMA-IR | Mehta et al.[40] |
| Cross-sectional | 2015 | PAEs | Urine | Averaged 12.8 weeks of gestation | USA | 72 | No association | Robledo et al.[66] |
| Cross-sectional | 2020 | PAEs | Meconium | At the first 24h after delivery | China | 251 | Positive association between MiBP [24.05 ng/g], MnBP [26.64 ng/g], MEHP [51.82 ng/g] and GDM in women with male fetusesa | Guo et al.[71] |
| Cross-sectional | 2022 | PAEs | Urine | 1st trimester | China | 200 | Higher MEHP [4.25 vs. 2.71 mu g/L] levels in GDM casesb | Liang et al.[75] |
| Case-control | 2016 | PAEs | Urine | Clinic visit 1, 2, 3, 4 | USA | 350 | Positive association between MEP and IGT Negative association between DEHP and IGT | James-Todd et al.[68] |
| Case-control | 2022 | PAEs | Urine | Visit 1, 2, 3, 4 | USA | 606 | 1st trimester: Negative association between MBP [11.60 ng/mL], MCNP [2.41 ng/mL], MCPP [2.31 ng/mL] and IGTa 2nd trimester: Negative association between MCNP [1.98 ng/mL], MCPP [1.90 ng/mL] and IGT; Positive association between MiBP [5.84 ng/mL], MHBP [1.23 ng/mL] and IGTa | James-Todd et al.[74] |
| Case-control | 2023 | PAEs | Serum | At the time of delivery | China | 201 | Positive association between MBP [3.52 vs. 3.37 ng/mL], MiBP [0.86 vs. 0.57 ng/mL] and 2h-PG, GDMb | Wang et al.[76] |
| Cohort | 2015 | PAEs | Urine | 1st trimester | Canada | 1,274 | No association | Shapiro et al.[67] |
| Cohort | 2018 | PAEs | Urine | 1st and 2nd trimester | USA | 245 | Positive association between MEP [60.2 ng/mL] and glucose levels Negative association between MiBP [5.7 ng/mL] and glucose levelsa,c | James-Todd et al.[69] |
| Cohort | 2019 | PAEs | Urine | 1st and 3rd pregnancy | USA | 705 | Positive association between T1T3avg MEP and GDM | Shaffer et al.[70] |
| Cohort | 2021 | PAEs | Urine | 1st, 2nd, 3rd trimester | China | 3,269 | Positive association with blood glucose, GDM | Gao et al.[72] |
| Cohort | 2021 | PAEs | Urine | 1st and 2nd trimester | USA | 315 | No association | Zukin et al.[73] |
| Cohort | 2024 | PAEs | Urine | 1st trimester | China | 725 | Positive association between MBZP and GDM | Guo et al.[77] |
| Cohort | 2024 | PAEs | Urine | 2nd trimester | USA | 298 | Positive association between DEHP and fasting glucose, fasting insulin, and HOMA2-IR | Peng et al.[7] |
Overall, the epidemiological evidence on the relationship between PCB exposure and GDM risk remains inconsistent. While some studies have identified a positive association, others have found no significant relationship. This suggests the need for more large-scale, multicenter studies to clarify the potential biological mechanisms and dose-response relationships of PCB exposure during pregnancy, a physiologically vulnerable period for endocrine disruption, in relation to the development of GDM.
Experimental studies and possible mechanisms
Experimental studies provide insight into potential biological mechanisms linking PCBs to glucose metabolic dysfunction. PCB-126, a coplanar PCB congener, may disrupt the crosstalk between adipose tissue and skeletal muscle, particularly under insulin-resistant conditions. One in vitro study used a co-culture model to examine how PCB-126 affects adipokine secretion from adipocytes, and whether these adipocyte-derived factors alter glucose metabolism and mitochondrial function in myotubes[60]. The study found that PCB-126 exposure led to increased adipokine secretion from IR adipocytes, impairing glucose uptake in co-cultured myotubes[60]. These results highlight the crucial role of adipose-to-muscle communication in mediating PCB-126-induced metabolic abnormalities, particularly under insulin-resistant conditions.
An in vivo study using female Institute of Cancer Research mice also investigated the effects of PCB-126 exposure during the nursing period on glucose tolerance and body composition in offspring[61]. The results showed that maternal PCB-126 exposure did not significantly alter short- or long-term body composition in offspring but did lead to impaired glucose tolerance (IGT) in early life[61]. Importantly, these impairments were transient and largely reversible as the offspring matured[61]. These findings suggest that PCB-126 may induce early-life metabolic disturbances, which could predispose offspring to long-term metabolic issues.
In summary, PCBs, particularly PCB-126, may disrupt glucose metabolism through multiple pathways, including adipose-to-muscle signaling dysfunction and early-life programming effects, especially under conditions of IR or during developmental windows of vulnerability.
PHTHALATES
Phthalates (PAEs) are among the most widely used plasticizers, with ortho-phthalates being the most common types, including diethyl phthalate (DEP), dibutyl phthalate (DBP), diisobutyl phthalate (DIBP), diisononyl phthalate (DINP), and di(2-ethylhexyl) phthalate (DEHP)[62]. Since their introduction in the 1930s, PAEs have been extensively applied in the plastics industry to enhance the flexibility and elasticity of rigid polymers[63]. They are commonly used as additives and solvents in pharmaceuticals, cosmetics, and personal care products[64]. Due to their ability to leach from products, PAEs are readily absorbed by humans and are metabolized into monoesters, which are primarily excreted in urine, though they can also be detected in other bodily fluids and tissues[65]. As endocrine disruptors, PAEs have been identified as potential risk factors for various diseases, including GDM, due to their ability to interfere with hormone signaling pathways that regulate glucose metabolism.
Epidemiological studies
A total of thirteen epidemiological studies have investigated the relationship between PAE exposure and GDM, including three cross-sectional studies, three case-control studies, and seven prospective cohort studies [Table 2][7,66-77]. Most of these studies focused on maternal urine samples to assess exposure levels to various PAEs and their metabolites, with exposure timing varying across studies, from early to late pregnancy. For example, a cross-sectional study by Guo et al. in China analyzed meconium samples collected within the first 24 h after birth to assess in utero PAE exposure and its association with GDM[71]. The study found a positive association between levels of Mono-isobutyl phthalate (MiBP), Mono-n-butyl phthalate (MnBP), and Mono (2-ethylhexyl) phthalate (MEHP) in meconium and GDM risk, particularly among women carrying male fetuses[71]. A recent case-control study by Wang et al. in China, which analyzed serum samples collected at delivery from 201 postpartum women, also reported positive associations between serum levels of MBP and MiBP and GDM occurrence, as well as elevated 2h-PG levels during the OGTT[76]. In contrast, a cohort study by Zukin et al. in the United States, which measured urinary phthalate metabolite levels during early and mid-pregnancy, found no significant association between PAE exposure and GDM risk[73].
Overall, the epidemiological evidence suggests that PAE exposure may be associated with maternal glucose dysregulation and increased GDM risk, though findings remain inconsistent. Variations in study design, sample types, timing of measurements, and geographical regions contribute to discrepancies. Future studies should focus on critical exposure windows, metabolite specificity, sex differences, and potential confounders to better clarify the relationship between PAE exposure and GDM risk.
Experimental studies and possible mechanisms
PAEs may influence key factors involved in GDM pathogenesis. For instance, tumor necrosis factor-alpha (TNF-α) plays a significant role in GDM development by promoting adipocyte lipolysis, reducing insulin sensitivity in peripheral tissues, and serving as a biomarker for IR during pregnancy[63].
To date, only one animal study has directly investigated the link between PAE exposure and GDM. In this study, Sprague-Dawley rats were exposed to a combination of DBP and streptozotocin, resulting in a physiologically relevant GDM model[78]. In both in vitro and in vivo studies, DBP exposure was found to downregulate the expression of FoxM1 via the phosphorylation of STAT1 (pSTAT1)[78]. This molecular alteration led to decreased β-cell viability and increased apoptosis, contributing to the development of GDM[78].
In summary, PAEs may promote GDM by interfering with inflammatory and transcriptional pathways, particularly through the TNF-α-pSTAT1-FoxM1 axis, which disrupts β-cell survival and insulin regulation.
BISPHENOLS
Bisphenol A (BPA) and its structural analogues, including bisphenol S (BPS), bisphenol F (BPF), bisphenol B (BPB), and tetrabromobisphenol A (TBBPA), are synthetically produced chemicals widely used in the manufacture of polycarbonate plastics and epoxy resins[79]. Due to their extensive application in consumer products such as food containers, baby bottles, thermal papers, and medical devices, bisphenols are ubiquitous in the environment and human exposure is virtually unavoidable[80]. These compounds are known endocrine disruptors and have been implicated in metabolic dysregulation, including disturbances in lipid and glucose homeostasis[81]. Once absorbed through ingestion, inhalation, or dermal contact, bisphenols can interfere with hormone signaling pathways, raising increasing concerns about their potential role in the development of GDM[82,83]. In response to regulatory restrictions on BPA, structurally similar substitutes such as BPS and BPF are increasingly used, though their safety remains unclear.
Epidemiological studies
In epidemiological studies investigating the association between bisphenols and GDM, nine studies have examined the relationship between BPA and GDM or glucose metabolism, while four studies have simultaneously assessed BPA and its analogs, such as BPS, BPB, and BPF[7,8,41,67,84-92]. A cross-sectional study conducted by Hou et al. involving 390 pregnant women in China assessed urinary BPA levels during 24-28 weeks of gestation but found no significant association with GDM[84]. In contrast, a case-control study conducted in the United States by Zhu et al. reported a significant positive association between first-trimester BPS exposure and GDM risk[85]. Moreover, elevated BPA levels were linked to increased GDM susceptibility in non-Asian/Pacific Islander populations[85]. Similarly, a recent Canadian cohort study identified a positive correlation between second-trimester BPA concentrations and GDM risk, supporting the hypothesis that bisphenols may exert endocrine-disrupting effects during pregnancy[8].
Despite some inconsistencies, evidence suggests that bisphenol-related GDM risk may vary by exposure timing, ethnicity, and individual susceptibility. As BPA use declines, its analogs are increasingly adopted, though their safety remains unclear, underscoring the need for improved toxicological assessment.
Experimental studies and possible mechanisms
Growing experimental evidence supports the role of bisphenols, especially BPA, as endocrine disruptors involved in GDM pathogenesis. A meta-analysis points out that BPA accumulates in adipose tissue, disrupts adipokine secretion, and induces IR, and it impairs insulin signaling via modulation of potassium channels and promotes β-cell apoptosis through mitochondrial damage[93]. Perinatal BPA exposure also increases diabetes risk in offspring, potentially mediated by epigenetic mechanisms such as altered DNA methylation, histone modification, and miRNA expression[93].
Animal studies have shown that gestational BPA exposure leads to postpartum glucose intolerance, IR, and weight gain. These effects are linked to impaired insulin secretion, reduced β-cell mass, downregulation of cyclin D2 and CDK4, and upregulation of cell cycle inhibitors such as p16 and p53[94]. Interestingly, such effects were absent in non-pregnant females, suggesting that pregnancy is a critical window of susceptibility[94].
With BPA increasingly replaced by alternatives, attention has turned to compounds such as BPS and BPF. A systematic review of 32 studies (25 in vitro, 7 in vivo) confirmed that BPS and BPF exhibit endocrine-disrupting activities similar to BPA, including estrogenic, androgenic, and anti-androgenic effects[95]. BPS mimics estradiol through membrane signaling pathways, thereby influencing cell proliferation and apoptosis, while both BPS and BPF have been shown to alter organ weights, reproductive function, and enzyme expression, collectively raising concerns that these BPA substitutes may pose hormonal risks comparable to those of BPA[95].
ORGANOCHLORINE PESTICIDES
Organochlorine pesticides (OCPs) are a class of persistent organic pollutants (POPs) and recognized environmental estrogens[96]. Representative compounds include dichlorodiphenyltrichloroethane (DDT), its metabolite 1,1-dichloro-2,2-bis (p-chlorophenyl) ethylene (DDE), endosulfan, hexachlorobenzene (HCB), and hexachlorocyclohexane (HCH)[96,97]. Due to their high lipophilicity, chemical stability, and resistance to degradation, OCPs tend to accumulate in biological tissues and undergo biomagnification through the food chain[98]. Although most OCPs have been banned or strictly regulated, they are still widely detected in soil, water, and human tissues, particularly in adipose tissue, breast milk, and the placenta[99]. While several epidemiological studies have explored the association between OCP exposure and GDM, toxicological evidence remains limited.
Epidemiological studies
To date, few studies have explored the association between OCP exposure and GDM, and existing findings remain inconsistent [Table 3][9,32,35,59,100,101]. Most prospective cohort studies assess OCP concentrations in maternal serum or plasma and involve diverse populations across multiple countries. For instance, a Danish cohort of 604 pregnant women found a positive association between DDT, DDE levels in serum and breast milk at 34 weeks and GDM risk[35]. Conversely, a United States-based study reported a negative association between early pregnancy HCB levels and GDM[9]. Other studies conducted in different populations have reported no significant associations[32,59,100,101].
Summary of epidemiological evidence on the association between BPA, OCPs, APs, and paraben exposure and GDM
| Study type | Year | Biological sample | Population | Findings (GM/Med) | Author | |||
| Analyte | Matrix | Gestational week | Country | Size | ||||
| Cross-sectional | 2021 | BPA | Urine | 24-28 weeks of gestation | China | 390 | No association BPA [0.58 μg/L]a | Hou et al.[84] |
| Case-control | 2013 | BPA | Urine | 2nd trimester | USA | 94 | No association BPA [0.80 vs. 1.67 μg/L]a,c | Robledo et al.[86] |
| Case-control | 2022 | BPA | Urine | 2nd trimester | USA | 301 | Negative association between BPA [1.85 vs. 2.58 ng/mL] and GDMa,c | Chen et al.[92] |
| Case-control | 2022 | BPA, BPS, BPF | Urine | 1st and/or 2nd trimester | USA | 333 | Positive association between BPS [0.6 vs. 0.4 ng/mL], BPA[non-A/PIs][0.6 vs. 0.5 ng/mL] and GDM in the 1st trimester | Zhu et al.[85] |
| Cohort | 2015 | BPA | Urine | 1st trimester | Canada | 1,274 | No association BPA [0.9 μg/L]a,c | Shapiro et al.[67] |
| Cohort | 2017 | BPA | Urine | 1st and/or 2nd trimester | USA | 245 | No association with blood glucose in 1st trimester BPA [1.39 μg/L]a,c Positive association with increased blood glucose in the 2nd trimester BPA [1.27 μg/L]a,c | Chiu et al.[87] |
| Cohort | 2017 | BPA | Urine | 3rd trimester | China | 620 | Negative association with 2h-PG and GDM BPA [2.72 μg/L]a,c | Wang et al.[88] |
| Cohort | 2018 | BPA | Urine | 1st and/or 2nd trimester | USA | 347 | No association BPA [1st:1.3; 2nd: 1.28 μg/L]a,c | Bellavia et al.[89] |
| Cohort | 2018 | BPA | Serum | 10-17 weeks of gestation | UK | 232 | No association BPA [1.76 μg/L]b | Fisher et al.[90] |
| Cohort | 2019 | BPA, BPS, BPF, BPAF | Urine | 1st trimester | China | 1,841 | Positive association between BPAF [0.030 μg/L] and GDMa,c Positive association between BPAF, BPS [0.36 μg/L] and FPGa,c | Zhang et al.[91] |
| Cohort | 2021 | BPA | Serum | 16 and 26 weeks of gestation | USA | 388 | No association BPA [16w 1.9; 26w 1.8 ng/mL]a | Vuong et al.[41] |
| Cohort | 2024 | BPA, BPS | Urine | 2nd trimester | Canada | 420 | Positive association between BPA [1.12 μg/L] and GDMa | Soomro et al.[8] |
| Cohort | 2024 | BPA, BPS | Urine | 2nd trimester | USA | 298 | No association | Peng et al.[7] |
| Cohort | 2014 | Chlordecone | Plasma | during labor | French | 779 | No association Chlordecone [0.4 μg/L]b | Saunders et al.[100] |
| Cohort | 2016 | OCPs | Plasma | 1st trimester | Canada | 1,274 | No association | Shapiro et al.[32] |
| Cohort | 2016 | OCPs | Serum | Before pregnancy | USA | 258 | No association | Smarr et al.[101] |
| Cohort | 2017 | HCB, p, p’DDE | Serum | 1st trimester | Greece | 939 | No association between HCB [103.8 vs. 86.5 pg/mL] and p, p’DDE [2067.9 vs. 2,041.1 pg/mL] and GDMa | Vafeiadi et al.[59] |
| Cohort | 2017 | DDE, DDT | Serum milk | 34 weeks of gestation | Denmark | 604 | Positive association between OCPs and GDM Serum DDE [0.54 μg/g lipid]; Serum DDT [0.00 μg/g lipid]b | Valvi et al.[35] |
| Cohort | 2019 | OCPs | Plasma | 8-13 weeks of gestation | USA | 2334 | Negative association between HCB [7.4 ng/g lipid] and GDMa,c | Rahman et al.[9] |
| Cross-sectional | 2025 | Parabens | Urine | 1st and 2nd trimesters | USA | 333 | Positive associations between PrP, MeP, and EtP and GDM in A/PI | Peterson et al.[10] |
| Cohort | 2019 | Parabens | Urine | 1st and 2nd trimesters | USA | 241 | Positive associations between BuP [1st: 0.94; 2nd: 1 μg/L] and glucose levels in 1st trimester and 2nd trimester Negative association between PrP [1st:23.2; 2nd: 25.3 μg/L] and glucose levels in 1st trimestera,c | Bellavia et al.[106] |
| Cohort | 2019 | Parabens | Urine | Within 3 days before or after delivery | China | 696 | Nonlinear associations of PrP [0.49 ng/mL] and the summed estrogenic activity of parabens with GDM in the overweight/obese populationa,c | Li et al.[107] |
| Cohort | 2019 | Parabens | Urine | 8-16 weeks of gestation | China | 1,087 | Positive association between EtP [0.83 vs. 0.53 μg/L] and GDMb,c | Liu et al.[108] |
| Cohort | 2024 | Parabens | Urine | 2nd trimester | USA | 298 | Lower IGT/GDM prevalence with detectable EtP | Peng et al.[7] |
| Cross-sectional | 2021 | NP, and 2-t-OP | Urine | 24-28 weeks of gestation | China | 390 | Positive association between 2-t-OP [1.80 μg/L] and GDM Negative association between NP [0.57 μg/L] and GDMa,c | Hou et al.[84] |
| Cohort | 2024 | NP, 4-n-NP, 4-t-OP, 4-n-OP | Serum | 1st trimester | China | 2,035 | Positive association between NP [107.1 ng/mL] and GDM; Positive association between 4-t-OP [45.91 ng/mL] and GDM in women with female fetuses U-shaped nonlinear association between 4-n-OP [0.92 ng/mL] and 4-n-NP [1.77 ng/mL] with GDM in women with female fetusesb | Pang et al.[11] |
Overall, current epidemiological evidence on the relationship between OCP exposure and GDM is inconclusive. The heterogeneity in outcomes, ranging from positive to null or inverse associations, may be influenced by differences in population characteristics, timing and type of sample collection, and specific OCP compounds assessed. These findings suggest that the metabolic impact of OCPs may vary depending on the exposure context and compound-specific properties.
PARABENS
Parabens, or alkyl esters of p-hydroxybenzoic acid, have been widely used as antimicrobial preservatives in pharmaceuticals, cosmetics, and food products since the 1930s[102]. Although they can be absorbed through the skin and other routes, parabens are rapidly metabolized to p-hydroxybenzoic acid and excreted in urine, resulting in a short biological half-life[103]. Consequently, their systemic toxicity is generally considered low. However, topical application on damaged skin may induce allergic reactions, and patch testing has shown delayed-type hypersensitivity at high concentrations (5%-15%) in sensitive individuals[104].
In recent years, the widespread use of parabens in moisturizers, sunscreens, and other personal care products has raised concerns over their potential role as emerging environmental pollutants. Trace levels of methylparaben (MeP), ethylparaben (EtP), and propylparaben (PrP) have been detected in breast milk, though concentrations remain well below the acceptable daily intake thresholds[105]. Research on parabens and GDM has largely focused on epidemiological studies, with limited toxicological evidence available.
Epidemiological studies
Epidemiological studies examining the association between paraben exposure and GDM are primarily concentrated in the United States and China, with most adopting prospective cohort designs and analyzing urinary biomarkers during pregnancy. To date, findings consistently suggest that paraben exposure is mostly positively associated with GDM risk [Table 3][7,10,106-108].
For example, a United States cross-sectional study assessed urinary concentrations of PrP, MeP, and EtP during the first and second trimesters, revealing significant positive associations with GDM, particularly among Asian/Pacific Islander women[10]. Similarly, a Chinese cohort study by Li et al. measured urinary paraben levels near delivery in 696 pregnant women and observed nonlinear associations between PrP and the combined estrogenic activity of paraben with GDM risk, especially in overweight or obese individuals[107].
Overall, evidence points toward a potentially elevated susceptibility to paraben-related GDM among specific subgroups, with maternal body weight and ethnicity acting as possible effect modifiers. Moreover, the observed non-linear relationships underscore the need to consider complex dose-response dynamics in future studies.
ALKYLPHENOLS
Alkylphenols, including nonylphenol (NP) and octylphenol (OP), are synthetic phenolic compounds with known endocrine-disrupting properties due to their structural similarity to estrogens[109]. They are commonly used as precursors or degradation products of alkylphenol ethoxylates, which serve as surfactants in industrial applications such as detergents, lubricants, pesticides, plastic additives, and textile processing[109,110]. Due to their widespread use, environmental persistence, and bioaccumulative potential, alkylphenols have been detected in water, soil, and biological matrices, with human exposure occurring via ingestion, inhalation, and dermal absorption[111].
Epidemiological studies
To date, only two epidemiological studies have examined the association between alkylphenol exposure and GDM, both conducted in China[11,84]. Hou et al. analyzed urinary concentrations of NP and 2-t-octylphenol (2-t-OP) in 390 pregnant women during the second trimester, identifying a positive association between 2-t-OP and GDM risk, while NP was inversely associated[84]. A larger cohort study of 2,035 pregnant women assessed multiple NP and OP homologs in early pregnancy serum and found that NP exposure was positively associated with GDM[11]. Furthermore, high exposure to 4-tert-Octylphenol significantly increased GDM risk, particularly among women carrying female fetuses[11].
These findings, although limited, suggest that alkylphenols may influence GDM risk in a compound-specific and potentially sex-dependent manner. However, the small number and geographic concentration of studies highlight the need for broader, multicenter investigations with more refined exposure assessments.
Experimental studies and possible mechanisms
Mechanistic studies specifically addressing alkylphenols and GDM remain scarce. However, research on T2DM and glucose metabolism offers relevant insights. For instance, in diabetic mice, exposure to xenoestrogens such as BPA and OP improved glycemic control and restored insulin secretion by upregulating β-cell transcription factors (Pdx1, Mafa, Neurod1), potentially via the NF-κB pathway[112]. These compounds also modulated hepatic gluconeogenesis, indicating broader metabolic effects.
Prenatal exposure to NP has been shown to induce hyperglycemia, increased body weight, and pancreatic inflammation in male offspring[113]. Mechanistically, NP disrupted the expression of glucose metabolism-related genes (e.g., GCK) and increased mitochondrial uncoupling protein UCP-2, suggesting interference with β-cell development and insulin synthesis[113].
Collectively, these studies implicate alkylphenols in multiple pathways relevant to glucose dysregulation, yet their specific contribution to GDM remains unclear. More targeted in vivo and in vitro research is needed to elucidate how alkylphenols influence gestational metabolic adaptation and disease susceptibility.
DISCUSSION
ECs are increasingly recognized as potential environmental risk factors for GDM. Although traditional risk factors for GDM include diet, obesity, and genetics, growing evidence suggests that prenatal exposure to ECs may also impair maternal glucose regulation. This review systematically integrates epidemiological studies on seven major classes of ECs, providing tabular summaries of the associations between different contaminants and GDM for direct comparison. In addition, the review synthesizes available evidence on potential mechanisms [Table 4], including inflammatory responses, oxidative stress, impaired insulin signaling, and gut microbiota dysbiosis, offering insights into the underlying pathways through which ECs may affect glucose metabolism.
Mechanistic overview of seven classes of ECs on GDM
| Mechanism | PFAS | PCBs | PAEs | Bisphenols | OCPs | Parabens | Alkyphenols |
| β-cell damage or insulin secretion decrease | ● | ○ | ● | ● | ○ | ○ | ● |
| Insulin resistance or tissue metabolism abnormality | ● | ● | ● | ● | ○ | ○ | ● |
| Inflammation or oxidative stress | ● | ○ | ● | ● | ○ | ○ | ● |
| Nuclear receptor or signaling disruption | ● | ○ | ● | ● | ○ | ● | ● |
| Developmental or early-life exposure | ● | ● | ○ | ● | ○ | ○ | ● |
Although the potential relationship between ECs and GDM has been systematically summarized, several limitations remain. First, most existing studies have methodological constraints, as they are predominantly cross-sectional or case-control in design, limiting causal inference. Longitudinal studies can better clarify temporal sequences between exposure and outcomes and support causal interpretations; however, they are costly, time-consuming, prone to loss to follow-up, and some only assess exposure at a single time point, which may fail to capture dynamic exposure levels across different stages of pregnancy. Second, exposure assessment methods remain insufficient. Most studies rely on single-point measurements in serum or urine, which may underestimate or overlook long-term low-dose and mixed exposures. In addition, variations in exposure assessment methods, timing of measurement, and choice of outcome indicators, along with differences in GDM diagnostic criteria, reduce the comparability and consistency of findings across studies. While some studies suggest that ECs may influence GDM through inflammation, oxidative stress, impaired insulin signaling, and gut microbiota alterations, mechanistic evidence for certain contaminant classes remains scarce. For example, parabens and organochlorine pesticides have limited experimental verification, with few systematic in vitro, in vivo, or animal model studies. Finally, there is substantial heterogeneity across studies. This heterogeneity may arise from differences in population genetics, dietary patterns, lifestyle, and environmental exposure profiles; variations in study design, sample size, exposure assessment methods, and confounding control; differences in contaminant levels and sources across regions; and inconsistent GDM diagnostic criteria. These factors likely influence the stability and comparability of results and partly explain inconsistencies among current findings.
In summary, existing evidence has limitations in study design, exposure assessment, mechanistic exploration, and result consistency. These gaps need to be addressed in future research. First, most current studies focus on a single contaminant or a single class of contaminants, whereas humans are generally exposed to mixtures of pollutants. Differences in sample types suitable for assessing different contaminants further complicate the situation. Therefore, future studies should strengthen research on mixed exposures, develop more comprehensive, precise, and efficient detection methods, and standardize exposure and outcome assessment protocols. Machine learning approaches, such as high-dimensional mixture models, could be applied to simulate real-world human exposures and further evaluate associated health risks. Second, at present, research on the effects of ECs on GDM is largely epidemiological, with relatively few studies based on animal models or in vitro/in vivo experiments. Future investigations could utilize the Adverse Outcome Pathway (AOP) framework[114] or network toxicology[115] to conduct risk assessments, integrate mechanistic information across different contaminants, and strengthen bidirectional validation between mechanistic and epidemiological evidence to improve the systematic understanding of pathogenic mechanisms.
In addition to the seven major classes of ECs summarized in this review, microplastics and pharmaceutical residues, which are more closely related to daily human life, also deserve attention. Microplastics are commonly present in the environment and may enter the human body through various pathways, such as drinking water, food, and air[116]. Evidence suggests that microplastics can induce inflammatory responses, oxidative stress, and gut microbiota dysbiosis, all of which may be closely linked to glucose metabolism disorders and GDM development[117,118]. Moreover, microplastics may serve as carriers for other toxic substances, potentially amplifying their effects[119]. However, evidence on the metabolic impacts of microplastics on pregnant populations remains limited, and future studies should combine epidemiological investigations with mechanistic research for deeper insights. Pharmaceutical residues primarily originate from improper use of antibiotics, hormones, and other drugs in daily life or from environmental contamination and can enter the human body via food, water, or environmental exposure[120]. Some residues possess endocrine-disrupting properties, potentially affecting glucose metabolism and insulin sensitivity directly or indirectly[121]. Given the regional variability of pharmaceutical residues, future research should clarify the risks associated with long-term low-dose exposure in pregnant women and fetuses and consider local dietary and environmental characteristics to inform food safety and public health policies.
CONCLUSION
ECs are increasingly recognized as potential environmental risk factors for GDM. While traditional contributors to GDM include diet, obesity, and genetics, growing evidence suggests that prenatal EC exposure may also impair maternal glucose regulation. However, current research remains limited in both epidemiological scope and mechanistic understanding. This review summarizes the associations between seven major classes of ECs and GDM, highlighting several key gaps: inconsistent findings across studies, limited temporal resolution of exposure assessments, and insufficient investigation of mixture effects. Mechanistic studies suggest that ECs may disrupt insulin signaling, induce oxidative stress, and impair β-cell function, yet such evidence is still lacking for some compounds such as parabens and organochlorine pesticides.
DECLARATIONS
Authors’ contributions
Study design: Li, H.; Wei, Y.; Peng, Y.
Data collection and analysis: Wei, Y.; Liu, M.; Wang, W.; Zou, X.
Data interpretation and manuscript preparation: Wei, Y.; Zhu, Z.; Zou, X.; Li, H.; Liu, M.; Peng, Y.
All authors reviewed and approved the final manuscript.
Availability of data and materials
Access to the trial data is restricted due to signed consent agreements on data sharing, which permit use only by external researchers conducting studies aligned with the objectives of this project. Researchers who wish to obtain the data used in this study can submit a request to the corresponding author.
Financial support and sponsorship
This work was funded by the National Key Research and Development Plan (2022YFE0132900), the Guangxi Science Fund for Distinguished Young Scholars (2024GXNSFFA010012), the National Natural Science Foundation of China (Nos. U22A20407 and 42167061), the Advanced Innovation Teams and Xinghu Scholars Program of Guangxi Medical University, and the Guangxi Key Laboratory of Reproductive Health and Birth Defect Prevention Open Subjects (No. GXWCHZDKF-2022-08).
Conflicts of interest
Han Li is a Junior Editorial Board member of Journal of Environmental Exposure Assessment. Han Li was not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author (s) 2025.
Declaration of generative AI use
During the preparation of this work, the authors used ChatGPT to improve language expression and check grammar. After using this tool, the authors reviewed and edited the content as needed and took full responsibility for the content of the publication.
Supplementary Materials
REFERENCES
1. Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: standards of care in diabetes-2025. Diabetes. Care. 2025, 48, S27-49.
2. International Diabetes Federation; Brussels, Belgium IDF Diabetes Atlas, 11th Ed. Available from: https://diabetesatlas.org (accessed on 2025-9-9).
3. Tuomi, T.; Santoro, N.; Caprio, S.; Cai, M.; Weng, J.; Groop, L. The many faces of diabetes: a disease with increasing heterogeneity. Lancet 2014, 383, 1084-94.
4. Lind, P. M.; Lind, L. Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia 2018, 61, 1495-502.
5. Yang, A.; Tam, C. H. T.; Wong, K. K.; et al. Epidemic-specific association of maternal exposure to per- and polyfluoroalkyl substances (PFAS) and their components with maternal glucose metabolism: a cross-sectional analysis in a birth cohort from Hong Kong. Sci. Total. Environ. 2024, 917, 170220.
6. Ma, J.; Li, Y.; Qian, L.; et al. Serum levels of polychlorinated biphenyls and polybrominated diphenyl ethers in early pregnancy and their associations with gestational diabetes mellitus. Chemosphere 2023, 339, 139640.
7. Peng, M. Q.; Dabelea, D.; Adgate, J. L.; et al. Associations of urinary biomarkers of phthalates, phenols, parabens, and organophosphate esters with glycemic traits in pregnancy: the healthy start study. Environ. Res. 2024, 262, 119810.
8. Soomro, M. H.; England-Mason, G.; Reardon, A. J. F.; et al.; APrON Study Team. Maternal exposure to bisphenols, phthalates, perfluoroalkyl acids, and trace elements and their associations with gestational diabetes mellitus in the APrON cohort. Reprod. Toxicol. 2024, 127, 108612.
9. Rahman, M. L.; Zhang, C.; Smarr, M. M.; et al. Persistent organic pollutants and gestational diabetes: a multi-center prospective cohort study of healthy US women. Environ. Int. 2019, 124, 249-58.
10. Peterson, A. K.; Zhu, Y.; Feng, J.; et al. Urinary concentrations of early and mid-pregnancy parabens and gestational diabetes: A nested case-control study within the PETALS cohort. Sci. Total. Environ. 2025, 974, 179253.
11. Pang, L.; Wei, H.; Wu, Y.; et al. Exposure to alkylphenols during early pregnancy and the risk of gestational diabetes mellitus: fetal sex-specific effects. Ecotoxicol. Environ. Saf. 2024, 287, 117270.
12. Murnyak, G.; Vandenberg, J.; Yaroschak, P. J.; Williams, L.; Prabhakaran, K.; Hinz, J. Emerging contaminants: presentations at the 2009 Toxicology and Risk Assessment Conference. Toxicol. Appl. Pharmacol. 2011, 254, 167-9.
13. Puri, M.; Gandhi, K.; Kumar, M. S. Emerging environmental contaminants: a global perspective on policies and regulations. J. Environ. Manage. 2023, 332, 117344.
14. Niu, H.; Xu, M.; Tu, P.; et al. Emerging contaminants: an emerging risk factor for diabetes mellitus. Toxics 2024, 12, 47.
15. Ouda, M.; Kadadou, D.; Swaidan, B.; et al. Emerging contaminants in the water bodies of the Middle East and North Africa (MENA): a critical review. Sci. Total. Environ. 2021, 754, 142177.
16. Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Perfluoroalkyls. Atlanta(GA): Agency for Toxic Substances and Disease Registry (US); 2021.
17. Gaines, L. G. T. Historical and current usage of per- and polyfluoroalkyl substances (PFAS): a literature review. Am. J. Ind. Med. 2023, 66, 353-78.
18. O’Hagan, D. Understanding organofluorine chemistry. an introduction to the C-F bond. Chem. Soc. Rev. 2008, 37, 308-19.
19. Domingo, J. L.; Nadal, M. Per- and polyfluoroalkyl substances (PFASs) in food and human dietary intake: a review of the recent scientific literature. J. Agric. Food. Chem. 2017, 65, 533-43.
20. Stein, C. R.; Wolff, M. S.; Calafat, A. M.; Kato, K.; Engel, S. M. Comparison of polyfluoroalkyl compound concentrations in maternal serum and amniotic fluid: a pilot study. Reprod. Toxicol. 2012, 34, 312-6.
21. Wang, Y.; Shi, Y.; Vestergren, R.; Zhou, Z.; Liang, Y.; Cai, Y. Using hair, nail and urine samples for human exposure assessment of legacy and emerging per- and polyfluoroalkyl substances. Sci. Total. Environ. 2018, 636, 383-91.
22. Domingo, J. L.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: a review of the recent scientific literature. Environ. Res. 2019, 177, 108648.
23. Li, J.; Yao, J.; Xia, W.; et al. Association between exposure to per- and polyfluoroalkyl substances and blood glucose in pregnant women. Int. J. Hyg. Environ. Health. 2020, 230, 113596.
24. Wang, Y.; Zhang, L.; Teng, Y.; et al. Association of serum levels of perfluoroalkyl substances with gestational diabetes mellitus and postpartum blood glucose. J. Environ. Sci. (China). 2018, 69, 5-11.
25. Xu, H.; Zhou, Q.; Zhang, J.; et al. Exposure to elevated per- and polyfluoroalkyl substances in early pregnancy is related to increased risk of gestational diabetes mellitus: a nested case-control study in Shanghai, China. Environ. Int. 2020, 143, 105952.
26. Liu, X.; Zhang, L.; Chen, L.; et al. Identification and prioritization of the potent components for combined exposure of multiple persistent organic pollutants associated with gestational diabetes mellitus. J. Hazard. Mater. 2021, 409, 124905.
27. Xu, C.; Zhang, L.; Zhou, Q.; et al. Exposure to per- and polyfluoroalkyl substances as a risk factor for gestational diabetes mellitus through interference with glucose homeostasis. Sci. Total. Environ. 2022, 838, 156561.
28. Peterson, A. K.; Zhu, Y.; Fuller, S.; et al. PFAS concentrations in early and mid-pregnancy and risk of gestational diabetes mellitus in a nested case-control study within the ethnically and racially diverse PETALS cohort. BMC. Pregnancy. Childbirth. 2023, 23, 657.
29. Zang, L.; Liu, X.; Xie, X.; Zhou, X.; Pan, Y.; Dai, J. Exposure to per- and polyfluoroalkyl substances in early pregnancy, risk of gestational diabetes mellitus, potential pathways, and influencing factors in pregnant women: a nested case-control study. Environ. Pollut. 2023, 326, 121504.
30. Zhang, Y.; Chen, R.; Gao, Y.; et al. Human serum poly- and perfluoroalkyl substance concentrations and their associations with gestational diabetes mellitus. Environ. Pollut. 2023, 317, 120833.
31. Zhang, C.; Sundaram, R.; Maisog, J.; Calafat, A. M.; Barr, D. B.; Buck, Louis. G. M. A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil. Steril. 2015, 103, 184-9.
32. Shapiro, G. D.; Dodds, L.; Arbuckle, T. E.; et al. Exposure to organophosphorus and organochlorine pesticides, perfluoroalkyl substances, and polychlorinated biphenyls in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: the MIREC study. Environ. Res. 2016, 147, 71-81.
33. Matilla-Santander, N.; Valvi, D.; Lopez-Espinosa, M. J.; et al. Exposure to perfluoroalkyl substances and metabolic outcomes in pregnant women: evidence from the Spanish INMA birth cohorts. Environ. Health. Perspect. 2017, 125, 117004.
34. Starling, A. P.; Adgate, J. L.; Hamman, R. F.; et al. Perfluoroalkyl substances during pregnancy and offspring weight and adiposity at birth: examining mediation by maternal fasting glucose in the healthy start study. Environ. Health. Perspect. 2017, 125, 067016.
35. Valvi, D.; Oulhote, Y.; Weihe, P.; et al. Gestational diabetes and offspring birth size at elevated environmental pollutant exposures. Environ. Int. 2017, 107, 205-15.
36. Jensen, R. C.; Glintborg, D.; Timmermann, C. A. G.; et al. Perfluoroalkyl substances and glycemic status in pregnant Danish women: the Odense child cohort. Environ. Int. 2018, 116, 101-7.
37. Wang, H.; Yang, J.; Du, H.; et al. Perfluoroalkyl substances, glucose homeostasis, and gestational diabetes mellitus in Chinese pregnant women: a repeat measurement-based prospective study. Environ. Int. 2018, 114, 12-20.
38. Preston, E. V.; Rifas-Shiman, S. L.; Hivert, M. F.; et al. Associations of per- and polyfluoroalkyl substances (PFAS) with glucose tolerance during pregnancy in project viva. J. Clin. Endocrinol. Metab. 2020, 105, e2864-76.
39. Ren, Y.; Jin, L.; Yang, F.; et al. Concentrations of perfluoroalkyl and polyfluoroalkyl substances and blood glucose in pregnant women. Environ. Health. 2020, 19, 88.
40. Mehta, S. S.; James-Todd, T.; Applebaum, K. M.; et al. Persistent organic pollutants and maternal glycemic outcomes in a diverse pregnancy cohort of overweight women. Environ. Res. 2021, 193, 110551.
41. Vuong, A. M.; Braun, J. M.; Sjödin, A.; et al. Exposure to endocrine disrupting chemicals (EDCs) and cardiometabolic indices during pregnancy: the HOME study. Environ. Int. 2021, 156, 106747.
42. Yu, G.; Jin, M.; Huang, Y.; et al.; Shanghai Birth Cohort Study. Environmental exposure to perfluoroalkyl substances in early pregnancy, maternal glucose homeostasis and the risk of gestational diabetes: a prospective cohort study. Environ. Int. 2021, 156, 106621.
43. Wang, Z.; Luo, J.; Zhang, Y.; et al.; Shanghai Birth Cohort. High maternal glucose exacerbates the association between prenatal per- and polyfluoroalkyl substance exposure and reduced birth weight. Sci. Total. Environ. 2023, 858, 160130.
44. Cinzori, M. E.; Pacyga, D. C.; Rosas, L.; et al. Associations of per- and polyfluoroalkyl substances with maternal metabolic and inflammatory biomarkers in early-to-mid-pregnancy. Environ. Res. 2024, 250, 118434.
45. Qin, W.; Ren, X.; Zhao, L.; Guo, L. Exposure to perfluorooctane sulfonate reduced cell viability and insulin release capacity of β cells. J. Environ. Sci. (China). 2022, 115, 162-72.
46. Zheng, F.; Sheng, N.; Zhang, H.; Yan, S.; Zhang, J.; Wang, J. Perfluorooctanoic acid exposure disturbs glucose metabolism in mouse liver. Toxicol. Appl. Pharmacol. 2017, 335, 41-8.
47. Wan, H. T.; Zhao, Y. G.; Leung, P. Y.; Wong, C. K. Perinatal exposure to perfluorooctane sulfonate affects glucose metabolism in adult offspring. PLoS. One. 2014, 9, e87137.
48. Yu, G.; Wang, J.; Liu, Y.; et al. Metabolic perturbations in pregnant rats exposed to low-dose perfluorooctanesulfonic acid: an integrated multi-omics analysis. Environ. Int. 2023, 173, 107851.
49. Yu, G.; Luo, T.; Liu, Y.; et al. Multi-omics reveal disturbance of glucose homeostasis in pregnant rats exposed to short-chain perfluorobutanesulfonic acid. Ecotoxicol. Environ. Saf. 2024, 278, 116402.
50. Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Polychlorinated Biphenyls (PCBs). Atlanta(GA): Agency for Toxic Substances and Disease Registry (US); 2000.
51. Liu, J.; Li, G.; Liu, J.; et al. Recent progress on toxicity and detection methods of polychlorinated biphenyls in environment and foodstuffs. Crit. Rev. Anal. Chem. 2023, 53, 928-53.
52. Us Epa, O. EPA Bans PCB Manufacture; Phases out Uses. Available from: https://www.epa.gov/archive/epa/aboutepa/epa-bans-pcb-manufacture-phases-out-uses.html (accessed on 2025-9-9).
53. Zhu, M.; Yuan, Y.; Yin, H.; et al. Environmental contamination and human exposure of polychlorinated biphenyls (PCBs) in China: a review. Sci. Total. Environ. 2022, 805, 150270.
54. Christensen, K.; Carlson, L. M.; Lehmann, G. M. The role of epidemiology studies in human health risk assessment of polychlorinated biphenyls. Environ. Res. 2021, 194, 110662.
55. Neblett, M. F. 2nd.; Curtis, S. W.; Gerkowicz, S. A.; et al. Examining reproductive health outcomes in females exposed to polychlorinated biphenyl and polybrominated biphenyl. Sci. Rep. 2020, 10, 3314.
56. Zhang, L.; Liu, X.; Meng, G.; et al. Non-dioxin-like polychlorinated biphenyls in early pregnancy and risk of gestational diabetes mellitus. Environ. Int. 2018, 115, 127-32.
57. Eslami, B.; Naddafi, K.; Rastkari, N.; Rashidi, B. H.; Djazayeri, A.; Malekafzali, H. Association between serum concentrations of persistent organic pollutants and gestational diabetes mellitus in primiparous women. Environ. Res. 2016, 151, 706-12.
58. Jaacks, L. M.; Barr, D. B.; Sundaram, R.; Maisog, J. M.; Zhang, C.; Buck, Louis. G. M. Pre-pregnancy maternal exposure to polybrominated and polychlorinated biphenyls and gestational diabetes: a prospective cohort study. Environ. Health. 2016, 15, 11.
59. Vafeiadi, M.; Roumeliotaki, T.; Chalkiadaki, G.; et al. Persistent organic pollutants in early pregnancy and risk of gestational diabetes mellitus. Environ. Int. 2017, 98, 89-95.
60. Caron, A.; Ahmed, F.; Peshdary, V.; Garneau, L.; Atlas, E.; Aguer, C. Effects of PCB126 on adipose-to-muscle communication in an in vitro model. Environ. Health. Perspect. 2020, 128, 107002.
61. Rice, B. B.; Sammons, K. W.; Ngo, Tenlep. S. Y.; et al. Exposure to PCB126 during the nursing period reversibly impacts early-life glucose tolerance. Front. Endocrinol. (Lausanne). 2023, 14, 1085958.
62. Mariana, M.; Feiteiro, J.; Verde, I.; Cairrao, E. The effects of phthalates in the cardiovascular and reproductive systems: a review. Environ. Int. 2016, 94, 758-76.
63. Mariana, M.; Cairrao, E. The relationship between phthalates and diabetes: a review. Metabolites 2023, 13, 746.
64. Geyer, R.; Jambeck, J. R.; Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782.
65. Chang, W. H.; Herianto, S.; Lee, C. C.; Hung, H.; Chen, H. L. The effects of phthalate ester exposure on human health: A review. Sci. Total. Environ. 2021, 786, 147371.
66. Robledo, C. A.; Peck, J. D.; Stoner, J.; et al. Urinary phthalate metabolite concentrations and blood glucose levels during pregnancy. Int. J. Hyg. Environ. Health. 2015, 218, 324-30.
67. Shapiro, G. D.; Dodds, L.; Arbuckle, T. E.; et al. Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: the MIREC study. Environ. Int. 2015, 83, 63-71.
68. James-Todd, T. M.; Meeker, J. D.; Huang, T.; et al. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ. Int. 2016, 96, 118-26.
69. James-Todd, T. M.; Chiu, Y. H.; Messerlian, C.; et al.; EARTH Study Team. Trimester-specific phthalate concentrations and glucose levels among women from a fertility clinic. Environ. Health. 2018, 17, 55.
70. Shaffer, R. M.; Ferguson, K. K.; Sheppard, L.; et al.; TIDES Study team. Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environ. Int. 2019, 123, 588-96.
71. Guo, J.; Wu, M.; Gao, X.; et al. Meconium exposure to phthalates, sex and thyroid hormones, birth size and pregnancy outcomes in 251 mother-infant Pairs from Shanghai. Int. J. Environ. Res. Public. Health. 2020, 17, 7711.
72. Gao, H.; Zhu, B. B.; Huang, K.; et al. Effects of single and combined gestational phthalate exposure on blood pressure, blood glucose and gestational weight gain: a longitudinal analysis. Environ. Int. 2021, 155, 106677.
73. Zukin, H.; Eskenazi, B.; Holland, N.; Harley, K. G. Prenatal exposure to phthalates and maternal metabolic outcomes in a high-risk pregnant Latina population. Environ. Res. 2021, 194, 110712.
74. James-Todd, T.; Ponzano, M.; Bellavia, A.; et al. Urinary phthalate and DINCH metabolite concentrations and gradations of maternal glucose intolerance. Environ. Int. 2022, 161, 107099.
75. Liang, Q. X.; Lin, Y.; Fang, X. M.; Gao, Y. H.; Li, F. Association between phthalate exposure in pregnancy and gestational diabetes: a Chinese cross-sectional study. Int. J. Gen. Med. 2022, 15, 179-89.
76. Wang, H.; Chen, R.; Gao, Y.; et al. Serum concentrations of phthalate metabolites in pregnant women and their association with gestational diabetes mellitus and blood glucose levels. Sci. Total. Environ. 2023, 857, 159570.
77. Guo, M.; Fang, Y.; Peng, M.; et al. Prenatal exposure to polycyclic aromatic hydrocarbons and phthalate acid esters and gestational diabetes mellitus: a prospective cohort study. Int. J. Hyg. Environ. Health. 2024, 261, 114419.
78. Chen, M.; Zhao, S.; Guo, W. H.; et al. Maternal exposure to Di-n-butyl phthalate (DBP) aggravate gestational diabetes mellitus via FoxM1 suppression by pSTAT1 signalling. Ecotoxicol. Environ. Saf. 2020, 205, 111154.
79. Michałowicz, J. Bisphenol A--sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738-58.
80. Sadrabad E, Hashemi SA, Nadjarzadeh A, Askari E, Akrami Mohajeri F, Ramroudi F. Bisphenol A release from food and beverage containers - a review. Food. Sci. Nutr. 2023, 11, 3718-28.
81. Ehrlich, S.; Lambers, D.; Baccarelli, A.; Khoury, J.; Macaluso, M.; Ho, S. M. Endocrine disruptors: a potential risk factor for gestational diabetes mellitus. Am. J. Perinatol. 2016, 33, 1313-8.
82. Rahmani, S.; Pour, Khalili. N.; Khan, F.; Hassani, S.; Ghafour-Boroujerdi, E.; Abdollahi, M. Bisphenol A: what lies beneath its induced diabetes and the epigenetic modulation? Life. Sci. 2018, 214, 136-44.
83. Buckley, J. P.; Kim, H.; Wong, E.; Rebholz, C. M. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013-2014. Environ. Int. 2019, 131, 105057.
84. Hou, Y.; Li, S.; Xia, L.; et al. Associations of urinary phenolic environmental estrogens exposure with blood glucose levels and gestational diabetes mellitus in Chinese pregnant women. Sci. Total. Environ. 2021, 754, 142085.
85. Zhu, Y.; Hedderson, M. M.; Calafat, A. M.; et al. Urinary phenols in early to midpregnancy and risk of gestational diabetes mellitus: a longitudinal study in a multiracial cohort. Diabetes 2022, 71, 2539-51.
86. Robledo, C.; Peck, J. D.; Stoner, J. A.; et al. Is bisphenol-A exposure during pregnancy associated with blood glucose levels or diagnosis of gestational diabetes? J. Toxicol. Environ. Health. A. 2013, 76, 865-73.
87. Chiu, Y. H.; Mínguez-Alarcón, L.; Ford, J. B.; et al.; for EARTH Study Team. Trimester-specific urinary bisphenol A concentrations and blood glucose levels among pregnant women from a fertility clinic. J. Clin. Endocrinol. Metab. 2017, 102, 1350-7.
88. Wang, X.; Wang, X.; Chen, Q.; et al. Urinary bisphenol a concentration and gestational diabetes mellitus in Chinese women. Epidemiology 2017, 28 Suppl 1, S41-7.
89. Bellavia, A.; Cantonwine, D. E.; Meeker, J. D.; et al. Pregnancy urinary bisphenol-A concentrations and glucose levels across BMI categories. Environ. Int. 2018, 113, 35-41.
90. Fisher, B. G.; Frederiksen, H.; Andersson, A. M.; et al. Serum phthalate and triclosan levels have opposing associations with risk factors for gestational diabetes mellitus. Front. Endocrinol. (Lausanne). 2018, 9, 99.
91. Zhang, W.; Xia, W.; Liu, W.; et al. Exposure to bisphenol a substitutes and gestational diabetes mellitus: a prospective cohort study in China. Front. Endocrinol. (Lausanne). 2019, 10, 262.
92. Chen, W. J.; Robledo, C.; Davis, E. M.; et al. Assessing urinary phenol and paraben mixtures in pregnant women with and without gestational diabetes mellitus: a case-control study. Environ. Res. 2022, 214, 113897.
93. Akash, M. S. H.; Sabir, S.; Rehman, K. Bisphenol A-induced metabolic disorders: from exposure to mechanism of action. Environ. Toxicol. Pharmacol. 2020, 77, 103373.
94. Alonso-Magdalena, P.; García-Arévalo, M.; Quesada, I.; Nadal, Á. Bisphenol-A treatment during pregnancy in mice: a new window of susceptibility for the development of diabetes in mothers later in life. Endocrinology 2015, 156, 1659-70.
95. Rochester, J. R.; Bolden, A. L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol a substitutes. Environ. Health. Perspect. 2015, 123, 643-50.
96. Kahn, L. G.; Philippat, C.; Nakayama, S. F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: implications for human health. Lancet. Diabetes. Endocrinol. 2020, 8, 703-18.
97. Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for DDT, DDE, and DDD. Atlanta(GA): Agency for Toxic Substances and Disease Registry (US); 2022.
98. Guo, W.; Pan, B.; Sakkiah, S.; et al. Persistent organic pollutants in food: contamination sources, health effects and detection methods. Int. J. Environ. Res. Public. Health. 2019, 16, 4361.
99. Keswani, C.; Dilnashin, H.; Birla, H.; et al. Global footprints of organochlorine pesticides: a pan-global survey. Environ. Geochem. Health. 2022, 44, 149-77.
100. Saunders, L.; Kadhel, P.; Costet, N.; et al. Hypertensive disorders of pregnancy and gestational diabetes mellitus among French Caribbean women chronically exposed to chlordecone. Environ. Int. 2014, 68, 171-6.
101. Smarr, M. M.; Grantz, K. L.; Zhang, C.; et al. Persistent organic pollutants and pregnancy complications. Sci. Total. Environ. 2016, 551-552, 285-91.
103. Fransway, A. F.; Fransway, P. J.; Belsito, D. V.; Yiannias, J. A. Paraben toxicology. Dermatitis 2019, 30, 32-45.
104. Soni, M. G.; Burdock, G. A.; Taylor, S. L.; Greenberg, N. A. Safety assessment of propyl paraben: a review of the published literature. Food. Chem. Toxicol. 2001, 39, 513-32.
105. Darbre, P. D.; Harvey, P. W. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. 2008, 28, 561-78.
106. Bellavia, A.; Chiu, Y. H.; Brown, F. M.; et al.; EARTH Study Team. Urinary concentrations of parabens mixture and pregnancy glucose levels among women from a fertility clinic. Environ. Res. 2019, 168, 389-96.
107. Li, Y.; Xu, S.; Li, Y.; et al. Association between urinary parabens and gestational diabetes mellitus across prepregnancy body mass index categories. Environ. Res. 2019, 170, 151-9.
108. Liu, W.; Zhou, Y.; Li, J.; et al. Parabens exposure in early pregnancy and gestational diabetes mellitus. Environ. Int. 2019, 126, 468-75.
109. Bergé, A.; Cladière, M.; Gasperi, J.; Coursimault, A.; Tassin, B.; Moilleron, R. Meta-analysis of environmental contamination by alkylphenols. Environ. Sci. Pollut. Res. Int. 2012, 19, 3798-819.
110. Ying, G. G.; Williams, B.; Kookana, R. Environmental fate of alkylphenols and alkylphenol ethoxylates--a review. Environ. Int. 2002, 28, 215-26.
111. Kovarova, J.; Blahova, J.; Divisova, L.; Svobodova, Z. Alkylphenol ethoxylates and alkylphenols--update information on occurrence, fate and toxicity in aquatic environment. Pol. J. Vet. Sci. 2013, 16, 763-72.
112. Kang, H. S.; Yang, H.; Ahn, C.; Kang, H. Y.; Hong, E. J.; Jaung, E. B. Effects of xenoestrogens on streptozotocin-induced diabetic mice. J. Physiol. Pharmacol. 2014, 65, 273-82.
113. Yang, J.; Yu, J.; Wang, P.; et al. The adverse effects of perinatal exposure to nonylphenol on carbohydrate metabolism in male offspring rats. Int. J. Environ. Health. Res. 2017, 27, 368-76.
114. Zhou, B. Adverse outcome pathway: framework, application, and challenges in chemical risk assessment. J. Environ. Sci. (China). 2015, 35, 191-3.
115. Hong, Y.; Wang, D.; Lin, Y.; et al. Environmental triggers and future risk of developing autoimmune diseases: Molecular mechanism and network toxicology analysis of bisphenol A. Ecotoxicol. Environ. Saf. 2024, 288, 117352.
116. Ali, N.; Katsouli, J.; Marczylo, E. L.; Gant, T. W.; Wright, S.; Bernardino, de. la. Serna. J. The potential impacts of micro-and-nano plastics on various organ systems in humans. EBioMedicine 2024, 99, 104901.
117. Hsiao, H. Y.; Nien, C. Y.; Shiu, R. F.; Chin, W. C.; Yen, T. H. Microplastic and nanoplastic exposure and risk of diabetes mellitus. World. J. Clin. Cases. 2025, 13, 98110.
118. Huang, D.; Zhang, Y.; Long, J.; et al. Polystyrene microplastic exposure induces insulin resistance in mice via dysbacteriosis and pro-inflammation. Sci. Total. Environ. 2022, 838, 155937.
119. Rafa, N.; Ahmed, B.; Zohora, F.; et al. Microplastics as carriers of toxic pollutants: Source, transport, and toxicological effects. Environ. Pollut. 2024, 343, 123190.
120. Liu, N.; Jin, X.; Feng, C.; et al. Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: a proposed multiple-level system. Environ. Int. 2020, 136, 105454.
121. Preston, E. V.; Lytel-Sternberg, J.; Quinn, M. R.; et al.; Environmental Reproductive and Glucose Outcomes (ERGO) Study. Associations of personal care product use during pregnancy and the postpartum period with markers of postpartum glycemic control - Results from the ERGO Study. Int. J. Hyg. Environ. Health. 2025, 266, 114569.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data




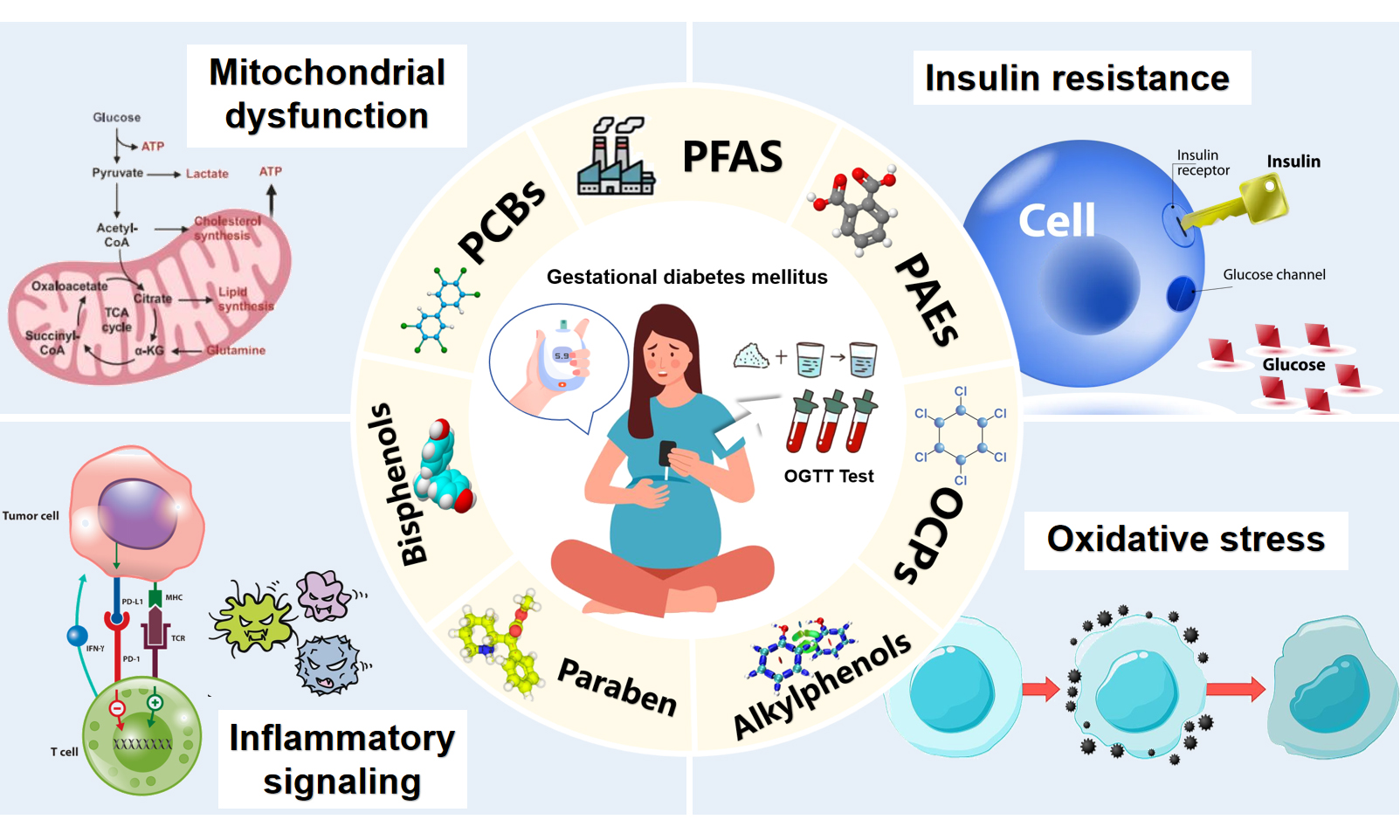











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.